EasySep™ Mouse CD4 Positive Selection Kit II
Immunomagnetic positive selection kit
概要
The EasySep™ Mouse CD4 Positive Selection Kit II is designed to isolate CD4+ cells from single-cell suspensions of splenocytes or other tissues by positive selection. Desired cells are targeted with antibody complexes recognizing CD4 and dextran-coated magnetic particles. Labeled cells are separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off.
This product replaces the EasySep™ Mouse CD4 Positive Selection Kit (Catalog #18752) for even faster cell isolations and does not result in the labeling of isolated cells with PE.
This product replaces the EasySep™ Mouse CD4 Positive Selection Kit (Catalog #18752) for even faster cell isolations and does not result in the labeling of isolated cells with PE.
Advantages
• Fast and easy-to-use
• Up to 99% purity
• No columns required
• Isolated cells are not fluorochrome-labeled
• Up to 99% purity
• No columns required
• Isolated cells are not fluorochrome-labeled
Components
- EasySep™ Mouse CD4 Positive Selection Kit II (Catalog #18952)
- EasySep™ Mouse CD4 Positive Selection II Component A, 0.5 mL
- EasySep™ Mouse CD4 Positive Selection II Component B, 0.5 mL
- Normal Rat Serum, 2 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Empty Vial
- RoboSep™ Mouse CD4 Positive Selection Kit II (Catalog #18952RF)
- EasySep™ Mouse CD4 Positive Selection II Component A, 0.5 mL
- EasySep™ Mouse CD4 Positive Selection II Component B, 0.5 mL
- Normal Rat Serum, 2 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Empty Vial
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125) x 2
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD4+
Species
Mouse
Sample Source
Other, Spleen
Selection Method
Positive
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse CD4 Positive Selection Kit II | 18952 | All | English |
| Product Information Sheet | RoboSep™ Mouse CD4 Positive Selection Kit II | 18952RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse CD4 Positive Selection Kit II | 18952 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse CD4 Positive Selection Kit II | 18952 | All | English |
| Safety Data Sheet 3 | EasySep™ Mouse CD4 Positive Selection Kit II | 18952 | All | English |
| Safety Data Sheet 4 | EasySep™ Mouse CD4 Positive Selection Kit II | 18952 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse CD4 Positive Selection Kit II | 18952RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse CD4 Positive Selection Kit II | 18952RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Mouse CD4 Positive Selection Kit II | 18952RF | All | English |
数据及文献
Data
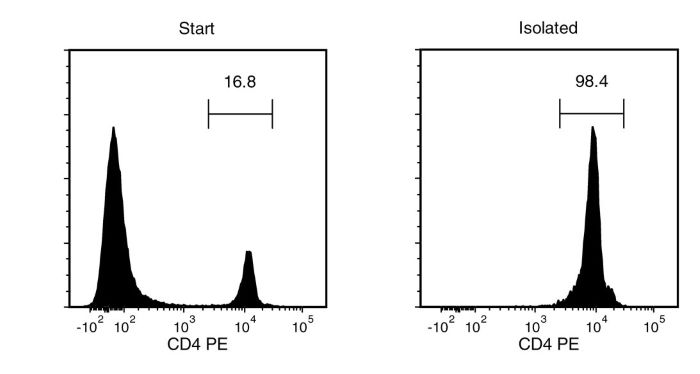
Figure 1. Typical EasySep™ CD4 Positive Selection Profile
Starting with mouse splenocytes, the CD4+ cell content of the isolated fraction is typically 98.6 ± 0.4% (mean ± SD for the purple EasySep™ Magnet).
Publications (4)
Nature communications 2020
T cell-intrinsic role for Nod2 in protection against Th17-mediated uveitis.
Abstract
Abstract
Mutations in nucleotide-binding oligomerization domain-containing protein 2 (NOD2) cause Blau syndrome, an inflammatory disorder characterized by uveitis. The antimicrobial functions of Nod2 are well-established, yet the cellular mechanisms by which dysregulated Nod2 causes uveitis remain unknown. Here, we report a non-conventional, T cell-intrinsic function for Nod2 in suppression of Th17 immunity and experimental uveitis. Reconstitution of lymphopenic hosts with Nod2-/- CD4+ T cells or retina-specific autoreactive CD4+ T cells lacking Nod2 reveals a T cell-autonomous, Rip2-independent mechanism for Nod2 in uveitis. In naive animals, Nod2 operates downstream of TCR ligation to suppress activation of memory CD4+ T cells that associate with an autoreactive-like profile involving IL-17 and Ccr7. Interestingly, CD4+ T cells from two Blau syndrome patients show elevated IL-17 and increased CCR7. Our data define Nod2 as a T cell-intrinsic rheostat of Th17 immunity, and open new avenues for T cell-based therapies for Nod2-associated disorders such as Blau syndrome.
Frontiers in immunology 2019
Macrophage Coordination of the Interferon Lambda Immune Response.
Abstract
Abstract
Lambda interferons (IFN-$\lambda$s) are a major component of the innate immune defense to viruses, bacteria, and fungi. In human liver, IFN-$\lambda$ not only drives antiviral responses, but also promotes inflammation and fibrosis in viral and non-viral diseases. Here we demonstrate that macrophages are primary responders to IFN-$\lambda$, uniquely positioned to bridge the gap between IFN-$\lambda$ producing cells and lymphocyte populations that are not intrinsically responsive to IFN-$\lambda$. While CD14+ monocytes do not express the IFN-$\lambda$ receptor, IFNLR1, sensitivity is quickly gained upon differentiation to macrophages in vitro. IFN-$\lambda$ stimulates macrophage cytotoxicity and phagocytosis as well as the secretion of pro-inflammatory cytokines and interferon stimulated genes that mediate immune cell chemotaxis and effector functions. In particular, IFN-$\lambda$ induced CCR5 and CXCR3 chemokines, stimulating T and NK cell migration, as well as subsequent NK cell cytotoxicity. Using immunofluorescence and cell sorting techniques, we confirmed that human liver macrophages expressing CD14 and CD68 are highly responsive to IFN-$\lambda$ ex vivo. Together, these data highlight a novel role for macrophages in shaping IFN-$\lambda$ dependent immune responses both directly through pro-inflammatory activity and indirectly by recruiting and activating IFN-$\lambda$ unresponsive lymphocytes.
Toxicology and applied pharmacology 2018 OCT
Astragaloside IV regulates differentiation and induces apoptosis of activated CD4+ T cells in the pathogenesis of experimental autoimmune encephalomyelitis.
Abstract
Abstract
CD4+ T cells, especially T-helper (Th) cells (Th1, Th2 and Th17) and regulatory T cells (Treg) play pivotal role in the pathogenesis of multiple sclerosis (MS), a demyelinating autoimmune disease occurring in central nervous system (CNS). Astragaloside IV (ASI, CAS: 84687-43-4) is one of the saponins isolated from Astragalus membranceus, a traditional Chinese medicine with immunomodulatory effect. So far, whether ASI has curative effect on experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and how it affects the subsets of CD4+ T cells, as well as the underlying mechanism have not been clearly elucidated. In the present study, ASI was found to ameliorate the progression and hamper the recurrence of EAE effectively in the treatment regimens. It significantly reduced the demyelination and inflammatory infiltration of CNS in EAE mice by suppressing the percentage of Th1 and Th17 cells, which was closely associated with the inhibition of JAK/STAT and NF-$\kappa$B signaling pathways. ASI also increased the percentage of Treg cells in spleen and CNS, which was accompanied by elevated Foxp3. However, in vitro experiments disclosed that ASI could regulate the differentiation of Th17 and Treg cells but not Th1 cells. In addition, it induced the apoptosis of MOG-stimulated CD4+ T cells probably through modulating STAT3/Bcl-2/Bax signaling pathways. Together, our findings suggested that ASI can modulate the differentiation of autoreactive CD4+ T cells and is a potential prodrug or drug for the treatment of MS and other similar autoimmune diseases.
Cell reports 2016 NOV
Opposing Development of Cytotoxic and Follicular Helper CD4 T Cells Controlled by the TCF-1-Bcl6 Nexus.
Abstract
Abstract
CD4(+) T cells develop distinct and often contrasting helper, regulatory, or cytotoxic activities. Typically a property of CD8(+) T cells, granzyme-mediated cytotoxic T cell (CTL) potential is also exerted by CD4(+) T cells. However, the conditions that induce CD4(+) CTLs are not entirely understood. Using single-cell transcriptional profiling, we uncover a unique signature of Granzyme B (GzmB)(+) CD4(+) CTLs, which distinguishes them from other CD4(+) T helper (Th) cells, including Th1 cells, and strongly contrasts with the follicular helper T (Tfh) cell signature. The balance between CD4(+) CTL and Tfh differentiation heavily depends on the class of infecting virus and is jointly regulated by the Tfh-related transcription factors Bcl6 and Tcf7 (encoding TCF-1) and by the expression of the inhibitory receptors PD-1 and LAG3. This unique profile of CD4(+) CTLs offers targets for their study, and its antagonism by the Tfh program separates CD4(+) T cells with either helper or killer functions.