EasySep™ Human Pan-DC Pre-Enrichment Kit
Immunomagnetic negative selection cell isolation kit
概要
The EasySep™ Human Pan-DC Pre-Enrichment Kit is designed to pre-enrich all dendritic cells (DCs) (including myeloid and plasmacytoid DCs) from fresh or previously frozen peripheral blood mononuclear cells by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-pan DCs and dextran-coated magnetic particles. The labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
Advantages
• Fast, easy-to-use and column-free
• Up to 80% purity
• Untouched, viable cells
• Up to 80% purity
• Untouched, viable cells
Components
- EasySep™ Human Pan-DC Pre-Enrichment Kit (Catalog #19251)
- EasySep™ Human Pan-DC Pre-Enrichment Cocktail Component A, 1 mL
- EasySep™ Human DC Enrichment Cocktail Component B, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- Anti-Human CD32 (Fc gamma RII) Blocker, 0.8 mL
- RoboSep™ Human Pan-DC Pre-Enrichment Kit with Filter Tips (Catalog #19251RF)
- EasySep™ Human Pan-DC Pre-Enrichment Cocktail Component A, 1 mL
- EasySep™ Human DC Enrichment Cocktail Component B, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- Anti-Human CD32 (Fc gamma RII) Blocker, 0.8 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Dendritic Cells
Species
Human
Sample Source
PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human Pan-DC Pre-Enrichment Kit | 19251 | All | English |
| Product Information Sheet | RoboSep™ Human Pan-DC Pre-Enrichment Kit with Filter Tips | 19251RF | All | English |
| Safety Data Sheet | EasySep™ Human Pan-DC Pre-Enrichment Kit | 19251 | All | English |
| Safety Data Sheet | RoboSep™ Human Pan-DC Pre-Enrichment Kit with Filter Tips | 19251RF | All | English |
数据及文献
Data
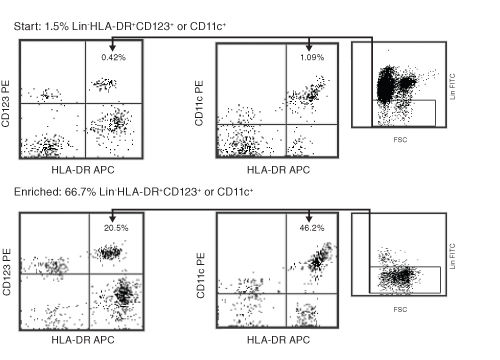
Figure 1. Typical Enrichment Profile For EasySep™ Human Pan-DC Pre-Enrichment Kit
Starting with previously frozen peripheral blood mononuclear cells, the dendritic cell content of the enriched fraction typically ranges from 40% - 80%.
Publications (3)
Journal of immunology (Baltimore, Md. : 1950) 2019 jul
Signaling Cascade through DC-ASGPR Induces Transcriptionally Active CREB for IL-10 Induction and Immune Regulation.
Abstract
Abstract
The types and magnitude of Ag-specific immune responses can be determined by the functional plasticity of dendritic cells (DCs). However, how DCs display functional plasticity and control host immune responses have not been fully understood. In this study, we report that ligation of DC-asialoglycoprotein receptor (DC-ASGPR), a C-type lectin receptor (CLR) expressed on human DCs, resulted in rapid activation of Syk, followed by PLCgamma2 and PKCdelta engagements. However, different from other Syk-coupled CLRs, including Dectin-1, signaling cascade through DC-ASGPR did not trigger NF-kappaB activation. Instead, it selectively activated MAPK ERK1/2 and JNK. Rapid and prolonged phosphorylation of ERK1/2 led to sequential activation of p90RSK and CREB, which consequently bound to IL10 promoter and initiated cytokine expression. In addition, DC-ASGPR ligation activated Akt, which differentially regulated the activities of GSK-3alpha/beta and beta-catenin and further contributed to IL-10 expression. Our observations demonstrate that DC-ASGPR induces IL-10 expression via an intrinsic signaling pathway, which provides a molecular explanation for DC-ASGPR-mediated programing of DCs to control host immune responses.
Nature immunology 2018 JAN
Diversification of human plasmacytoid predendritic cells in response to a single stimulus.
Abstract
Abstract
Innate immune cells adjust to microbial and inflammatory stimuli through a process termed environmental plasticity, which links a given individual stimulus to a unique activated state. Here, we report that activation of human plasmacytoid predendritic cells (pDCs) with a single microbial or cytokine stimulus triggers cell diversification into three stable subpopulations (P1-P3). P1-pDCs (PD-L1+CD80-) displayed a plasmacytoid morphology and specialization for type I interferon production. P3-pDCs (PD-L1-CD80+) adopted a dendritic morphology and adaptive immune functions. P2-pDCs (PD-L1+CD80+) displayed both innate and adaptive functions. Each subpopulation expressed a specific coding- and long-noncoding-RNA signature and was stable after secondary stimulation. P1-pDCs were detected in samples from patients with lupus or psoriasis. pDC diversification was independent of cell divisions or preexisting heterogeneity within steady-state pDCs but was controlled by a TNF autocrine and/or paracrine communication loop. Our findings reveal a novel mechanism for diversity and division of labor in innate immune cells.
Blood 2014 OCT
Human blood BDCA-1 dendritic cells differentiate into Langerhans-like cells with thymic stromal lymphopoietin and TGF-β.
Abstract
Abstract
The ontogeny of human Langerhans cells (LCs) remains poorly characterized, in particular the nature of LC precursors and the factors that may drive LC differentiation. Here we report that thymic stromal lymphopoietin (TSLP), a keratinocyte-derived cytokine involved in epithelial inflammation, cooperates with transforming growth factor (TGF)-β for the generation of LCs. We show that primary human blood BDCA-1(+), but not BDCA-3(+), dendritic cells (DCs) stimulated with TSLP and TGF-β harbor a typical CD1a(+)Langerin(+) LC phenotype. Electron microscopy established the presence of Birbeck granules, an intracellular organelle specific to LCs. LC differentiation was not observed from tonsil BDCA-1(+) and BDCA-3(+) subsets. TSLP + TGF-β LCs had a mature phenotype with high surface levels of CD80, CD86, and CD40. They induced a potent CD4(+) T-helper (Th) cell expansion and differentiation into Th2 cells with increased production of tumor necrosis factor-α and interleukin-6 compared with CD34-derived LCs. Our findings establish a novel LC differentiation pathway from BDCA-1(+) blood DCs with potential implications in epithelial inflammation. Therapeutic targeting of TSLP may interfere with tissue LC repopulation from circulating precursors.



