EasySep™ Human Naïve B Cell Enrichment Kit
Immunomagnetic negative selection kit
概要
The EasySep™ Human Naïve B Cell Enrichment Kit is designed to isolate naïve B cells from peripheral blood mononuclear cells by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-naïve B cells and dextran-coated magnetic particles. The labeled cells are separated using the EasySep™ magnet without the use of columns. The desired cells are poured off into a new tube.
Advantages
• Fast, easy-to-use and column-free
• Up to 98% purity
• Isolated cells are untouched
• Up to 98% purity
• Isolated cells are untouched
Components
- EasySep™ Human Naïve B Cell Enrichment Kit (Catalog #19254)
- EasySep™ Human Naïve B Cell Enrichment Cocktail, 1 mL
- EasySep™ Magnetic Particles, 5 x 1 mL
- RoboSep™ Human Naïve B Cell Enrichment Kit with Filter Tips (Catalog #19254RF)
- EasySep™ Human Naïve B Cell Enrichment Cocktail, 1 mL
- EasySep™ Magnetic Particles, 5 x 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
B Cells
Species
Human
Sample Source
Leukapheresis, PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human Naïve B Cell Enrichment Kit | 19254 | All | English |
| Product Information Sheet | RoboSep™ Human Naïve B Cell Enrichment Kit with Filter Tips | 19254RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human Naïve B Cell Enrichment Kit | 19254 | All | English |
| Safety Data Sheet 2 | EasySep™ Human Naïve B Cell Enrichment Kit | 19254 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human Naïve B Cell Enrichment Kit with Filter Tips | 19254RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human Naïve B Cell Enrichment Kit with Filter Tips | 19254RF | All | English |
数据及文献
Data
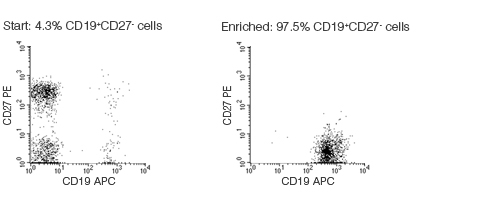
Figure 1. FACS Profile Results With EasySep™ Human Naïve B Cell Enrichment Kit
Starting with fresh mononuclear cells, the naïve B cell (CD19+CD27-) content of the isolated fraction typically ranges from 92 - 98%. In the above example, the purities of the start and isolated fractions are 4.3% and 97.5%, respectively.
Publications (5)
Leukemia 2020 may
CRL3-SPOP ubiquitin ligase complex suppresses the growth of diffuse large B-cell lymphoma by negatively regulating the MyD88/NF-$\kappa$B signaling.
Abstract
Abstract
Recurrent oncogenic mutations of MyD88 have been identified in a variety of lymphoid malignancies. Gain-of-function mutations of MyD88 constitutively activate downstream NF-$\kappa$B signaling pathways, resulting in increased cellular proliferation and survival. However, whether MyD88 activity can be aberrantly regulated in MyD88-wild-type lymphoid malignancies remains poorly understood. SPOP is an adaptor protein of CUL3-based E3 ubiquitin ligase complex and frequently mutated genes in prostate and endometrial cancers. In this study, we reveal that SPOP binds to and induces the nondegradative ubiquitination of MyD88 by recognizing an atypical SPOP-binding motif in MyD88. This modification blocks Myddosome assembly and downstream NF-$\kappa$B activation. SPOP is mutated in a subset of lymphoid malignancies, including diffuse large B-cell lymphoma (DLBCL). Lymphoid malignancies-associated SPOP mutants exhibited impaired binding to MyD88 and suppression of NF-$\kappa$B activation. The DLBCL-associated, SPOP-binding defective mutants of MyD88 escaped from SPOP-mediated ubiquitination, and their effect on NF-$\kappa$B activation is stronger than that of wild-type MyD88. Moreover, SPOP suppresses DLBCL cell growth in vitro and tumor xenograft in vivo by inhibiting the MyD88/NF-$\kappa$B signaling. Therefore, SPOP acts as a tumor suppressor in DLBCL. Mutations in the SPOP-MyD88 binding interface may disrupt the SPOP-MyD88 regulatory axis and promote aberrant MyD88/NF-$\kappa$B activation and cell growth in DLCBL.
Science advances 2020
B cell Sirt1 deacetylates histone and non-histone proteins for epigenetic modulation of AID expression and the antibody response.
Abstract
Abstract
Activation-induced cytidine deaminase (AID) mediates immunoglobulin class switch DNA recombination (CSR) and somatic hypermutation (SHM), critical processes for maturation of the antibody response. Epigenetic factors, such as histone deacetylases (HDACs), would underpin B cell differentiation stage-specific AID expression. Here, we showed that NAD+-dependent class III HDAC sirtuin 1 (Sirt1) is highly expressed in resting B cells and down-regulated by stimuli inducing AID. B cell Sirt1 down-regulation, deprivation of NAD+ cofactor, or genetic Sirt1 deletion reduced deacetylation of Aicda promoter histones, Dnmt1, and nuclear factor-$\kappa$B (NF-$\kappa$B) p65 and increased AID expression. This promoted class-switched and hypermutated T-dependent and T-independent antibody responses or led to generation of autoantibodies. Genetic Sirt1 overexpression, Sirt1 boost by NAD+, or allosteric Sirt1 enhancement by SRT1720 repressed AID expression and CSR/SHM. By deacetylating histone and nonhistone proteins (Dnmt1 and NF-$\kappa$B p65), Sirt1 transduces metabolic cues into epigenetic changes to play an important B cell-intrinsic role in modulating antibody and autoantibody responses.
Nature communications 2020
B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids.
Abstract
Abstract
Short-chain fatty acids (SCFAs) butyrate and propionate are metabolites from dietary fiber's fermentation by gut microbiota that can affect differentiation or functions of T cells, macrophages and dendritic cells. We show here that at low doses these SCFAs directly impact B cell intrinsic functions to moderately enhance class-switch DNA recombination (CSR), while decreasing at higher doses over a broad physiological range, AID and Blimp1 expression, CSR, somatic hypermutation and plasma cell differentiation. In human and mouse B cells, butyrate and propionate decrease B cell Aicda and Prdm1 by upregulating select miRNAs that target Aicda and Prdm1 mRNA-3'UTRs through inhibition of histone deacetylation (HDAC) of those miRNA host genes. By acting as HDAC inhibitors, not as energy substrates or through GPR-engagement signaling in these B cell-intrinsic processes, these SCFAs impair intestinal and systemic T-dependent and T-independent antibody responses. Their epigenetic impact on B cells extends to inhibition of autoantibody production and autoimmunity in mouse lupus models.
Molecular therapy. Methods {\&} clinical development 2019 sep
Enhancing Lentiviral and Alpharetroviral Transduction of Human Hematopoietic Stem Cells for Clinical Application.
Abstract
Abstract
Ex vivo retroviral gene transfer into CD34+ hematopoietic stem and progenitor cells (HSPCs) has demonstrated remarkable clinical success in gene therapy for monogenic hematopoietic disorders. However, little attention has been paid to enhancement of culture and transduction conditions to achieve reliable effects across patient and disease contexts and to maximize potential vector usage and reduce treatment cost. We systematically tested three HSPC culture media manufactured to cGMP and eight previously described transduction enhancers (TEs) to develop a state-of-the-art clinically applicable protocol. Six TEs enhanced lentiviral (LV) and five TEs facilitated alpharetroviral (ARV) CD34+ HSPC transduction when used alone. Combinatorial TE application tested with LV vectors yielded more potent effects, with up to a 5.6-fold increase in total expression of a reporter gene and up to a 3.8-fold increase in VCN. Application of one of the most promising combinations, the poloxamer LentiBOOST and protamine sulfate, for GMP-compliant manufacturing of a clinical-grade advanced therapy medicinal product (ATMP) increased total VCN by over 6-fold, with no major changes in global gene expression profiles or inadvertent loss of CD34+CD90+ HSPC populations. Application of these defined culture and transduction conditions is likely to significantly improve ex vivo gene therapy manufacturing protocols for HSPCs and downstream clinical efficacy.
Journal of Immunology 2016 APR
Shortened Intervals during Heterologous Boosting Preserve Memory CD8 T Cell Function but Compromise Longevity.
Abstract
Abstract
Developing vaccine strategies to generate high numbers of Ag-specific CD8 T cells may be necessary for protection against recalcitrant pathogens. Heterologous prime-boost-boost immunization has been shown to result in large quantities of functional memory CD8 T cells with protective capacities and long-term stability. Completing the serial immunization steps for heterologous prime-boost-boost can be lengthy, leaving the host vulnerable for an extensive period of time during the vaccination process. We show in this study that shortening the intervals between boosting events to 2 wk results in high numbers of functional and protective Ag-specific CD8 T cells. This protection is comparable to that achieved with long-term boosting intervals. Short-boosted Ag-specific CD8 T cells display a canonical memory T cell signature associated with long-lived memory and have identical proliferative potential to long-boosted T cells Both populations robustly respond to antigenic re-exposure. Despite this, short-boosted Ag-specific CD8 T cells continue to contract gradually over time, which correlates to metabolic differences between short- and long-boosted CD8 T cells at early memory time points. Our studies indicate that shortening the interval between boosts can yield abundant, functional Ag-specific CD8 T cells that are poised for immediate protection; however, this is at the expense of forming stable long-term memory.