EasySep™ Human Monocyte Isolation Kit
12.5-Minute cell isolation kit using immunomagnetic negative selection
概要
The EasySep™ Human Monocyte Isolation Kit is designed to isolate CD14+CD16- monocytes from fresh or previously frozen peripheral blood mononuclear cells or washed leukapheresis samples by immunomagnetic negative selection. The EasySep™ procedure involves labeling unwanted cells and platelets with antibody complexes and magnetic particles. The magnetically labelled cells are separated from the untouched desired cells by using an EasySep™ magnet and simply pouring or pipetting the desired cells into a new tube.
This product can be used in place of the EasySep™ Human Monocyte Enrichment Kit (Catalog #19059) for even faster cell isolations with reduced platelet contamination.
This product can be used in place of the EasySep™ Human Monocyte Enrichment Kit (Catalog #19059) for even faster cell isolations with reduced platelet contamination.
Advantages
• Fast, easy-to-use and column-free
• Up to 94% purity with high recovery
• Untouched, viable cells
• Up to 94% purity with high recovery
• Untouched, viable cells
Components
- EasySep™ Human Monocyte Isolation Kit (Catalog #19359)
- EasySep™ Human Monocyte Isolation Cocktail, 1 mL
- EasySep™ Human Platelet Removal Cocktail, 1 mL
- EasySep™ D Magnetic Particles for Human Monocytes, 1 mL
- RoboSep™ Human Monocyte Isolation Kit (Catalog #19359RF)
- EasySep™ Human Monocyte Isolation Cocktail, 1 mL
- EasySep™ Human Platelet Removal Cocktail, 1 mL
- EasySep™ D Magnetic Particles for Human Monocytes, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog #18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™ (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Monocytes
Species
Human
Sample Source
PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human Monocyte Isolation Kit | 19359 | All | English |
| Product Information Sheet | RoboSep™ Human Monocyte Isolation Kit | 19359RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human Monocyte Isolation Kit | 19359 | All | English |
| Safety Data Sheet 2 | EasySep™ Human Monocyte Isolation Kit | 19359 | All | English |
| Safety Data Sheet 3 | EasySep™ Human Monocyte Isolation Kit | 19359 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human Monocyte Isolation Kit | 19359RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human Monocyte Isolation Kit | 19359RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Human Monocyte Isolation Kit | 19359RF | All | English |
数据及文献
Data
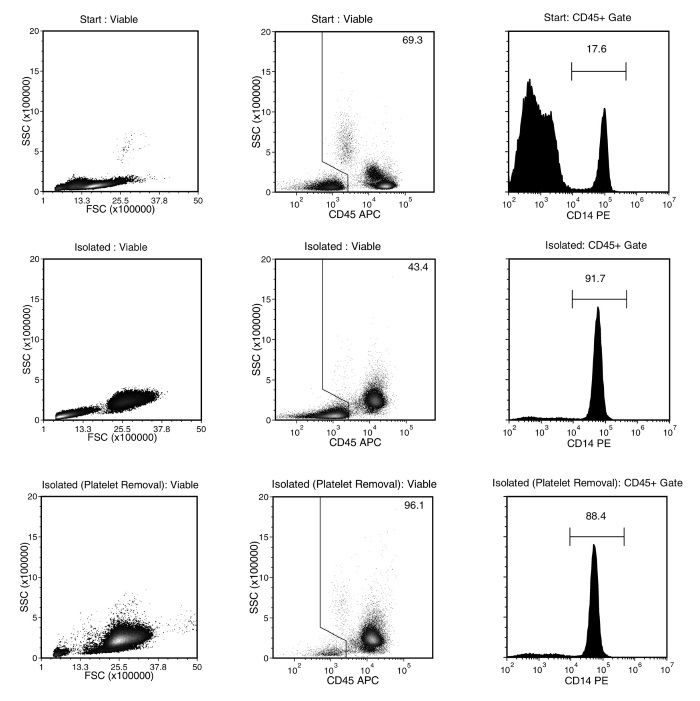
Figure 1. Typical EasySep™ Human Monocyte Isolation Profile
Starting with PBMCs prepared from human whole peripheral blood, the monocyte cell content (CD14+CD45+) of the isolated fraction obtained without (middle plots) or with EasySep™ Human Platelet Removal Cocktail (bottom plots) is typically 89.7 ± 3.4% and 87.3 ± 4.5%, respectively (gated on CD45, mean ± SD for the purple EasySep™ Magnet). In the above example, the purities of the start and final isolated fractions obtained without (middle plots) or with the EasySep™ Human Platelet Removal Cocktail (bottom plots) are 17.6%, 91.7% and 88.4%, respectively (gated on CD45) and 12.2%, 39.8% and 85.0% (not gated on CD45).
Publications (14)
Science advances 2020 may
Efficient blockade of locally reciprocated tumor-macrophage signaling using a TAM-avid nanotherapy.
Abstract
Abstract
Interpreting how multicellular interactions in the tumor affect resistance pathways to BRAF and MEK1/2 MAPK inhibitors (MAPKi) remains a challenge. To investigate this, we profiled global ligand-receptor interactions among tumor and stromal/immune cells from biopsies of MAPK-driven disease. MAPKi increased tumor-associated macrophages (TAMs) in some patients, which correlated with poor clinical response, and MAPKi coamplified bidirectional tumor-TAM signaling via receptor tyrosine kinases (RTKs) including AXL, MERTK, and their ligand GAS6. In xenograft tumors, intravital microscopy simultaneously monitored in situ single-cell activities of multiple kinases downstream of RTKs, revealing MAPKi increased TAMs and enhanced bypass signaling in TAM-proximal tumor cells. As a proof-of-principle strategy to block this signaling, we developed a multi-RTK kinase inhibitor nanoformulation that accumulated in TAMs and delayed disease progression. Thus, bypass signaling can reciprocally amplify across nearby cell types, offering new opportunities for therapeutic design.
Frontiers in immunology 2020
Inhibitory Effects of Dietary N-Glycans From Bovine Lactoferrin on Toll-Like Receptor 8; Comparing Efficacy With Chloroquine.
Abstract
Abstract
Toll-like receptor 8 (TLR-8) plays a role in the pathogenesis of autoimmune disorders and associated gastrointestinal symptoms that reduce quality of life of patients. Dietary interventions are becoming more accepted as mean to manage onset, progression, and treatment of a broad spectrum of inflammatory conditions. In this study, we assessed the impact of N-glycans derived from bovine lactoferrin (bLF) on the inhibition of TLR-8 activation. We investigated the effects of N-glycans in their native form, as well as in its partially demannosylated and partially desialylated form, on HEK293 cells expressing TLR-8, and in human monocyte-derived dendritic cells (MoDCs). We found that in HEK293 cells, N-glycans strongly inhibited the ssRNA40 induced TLR-8 activation but to a lesser extent the R848 induced TLR-8 activation. The impact was compared with a pharmaceutical agent, i.e., chloroquine (CQN), that is clinically applied to antagonize endosomal TLR- activation. Inhibitory effects of the N-glycans were not influenced by the partially demannosylated or partially desialylated N-glycans. As the difference in charge of the N-glycans did not influence the inhibition capacity of TLR-8, it is possible that the inhibition mediated by the N-glycans is a result of a direct interaction with the receptor rather than a result of pH changes in the endosome. The inhibition of TLR-8 in MoDCs resulted in a significant decrease of IL-6 when cells were treated with the unmodified (0.5-fold, p {\textless} 0.0001), partially demannosylated (0.3-fold, p {\textless} 0.0001) and partially desialylated (0.4-fold, p {\textless} 0.0001) N-glycans. Furthermore, the partially demannosylated and partially desialylated N-glycans showed stronger inhibition of IL-6 production compared with the native N-glycans. This provides evidence that glycan composition plays a role in the immunomodulatory activity of the isolated N-glycans from bLF on MoDCs. Compared to CQN, the N-glycans are specific inhibitors of TLR-8 activation and of IL-6 production in MoDCs. Our findings demonstrate that isolated N-glycans from bLF have attenuating effects on TLR-8 induced immune activation in HEK293 cells and human MoDCs. The inhibitory capacity of N-glycans isolated from bLF onTLR-8 activation may become a food-based strategy to manage autoimmune, infections or other inflammatory disorders.
Clinical cancer research : an official journal of the American Association for Cancer Research 2019 may
Anti-CD105 Antibody Eliminates Tumor Microenvironment Cells and Enhances Anti-GD2 Antibody Immunotherapy of Neuroblastoma with Activated Natural Killer Cells.
Abstract
Abstract
Purpose: We determined whether elimination of CD105+ cells in the tumor microenvironment (TME) with anti-CD105 antibodies enhanced anti-disialoganglioside (GD2) antibody dinutuximab therapy of neuroblastoma when combined with activated natural killer (aNK) cells.Experimental Design: The effect of MSCs and monocytes on antibody-dependent cellular cytotoxicity (ADCC) mediated by dinutuximab with aNK cells against neuroblastoma cells was determined in vitro. ADCC with anti-CD105 mAb TRC105 and aNK cells against MSCs, monocytes, and endothelial cells, which express CD105, was evaluated. Anti-neuroblastoma activity in immunodeficient NSG mice of dinutuximab with aNK cells without or with anti-CD105 mAbs was determined using neuroblastoma cell lines and a patient-derived xenograft.Results: ADCC mediated by dinutuximab with aNK cells against neuroblastoma cells in vitro was suppressed by addition of MSCs and monocytes, and dinutuximab with aNK cells was less effective against neuroblastomas formed with coinjected MSCs and monocytes in NSG mice than against those formed by tumor cells alone. Anti-CD105 antibody TRC105 with aNK cells mediated ADCC against MSCs, monocytes, and endothelial cells. Neuroblastomas formed in NSG mice by two neuroblastoma cell lines or a patient-derived xenograft coinjected with MSCs and monocytes were most effectively treated with dinutuximab and aNK cells when anti-human (TRC105) and anti-mouse (M1043) CD105 antibodies were added, which depleted human MSCs and murine endothelial cells and macrophages from the TME.Conclusions: Immunotherapy of neuroblastoma with anti-GD2 antibody dinutuximab and aNK cells is suppressed by CD105+ cells in the TME, but suppression is overcome by adding anti-CD105 antibodies to eliminate CD105+ cells.
Mucosal immunology 2019 jul
TNFalpha promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium.
Abstract
Abstract
Pathobiology of several chronic inflammatory disorders, including ulcerative colitis and Crohn's disease is related to intermittent, spontaneous injury/ulceration of mucosal surfaces. Disease morbidity has been associated with pathologic release of the pro-inflammatory cytokine tumor necrosis factor alpha (TNFalpha). In this report, we show that TNFalpha promotes intestinal mucosal repair through upregulation of the GPCR platelet activating factor receptor (PAFR) in the intestinal epithelium. Platelet activating factor (PAF) was increased in healing mucosal wounds and its engagement with epithelial PAFR leads to activation of epidermal growth factor receptor, Src and Rac1 signaling to promote wound closure. Consistent with these findings, delayed colonic mucosal repair was observed after administration of a neutralizing TNFalpha antibody and in mice lacking PAFR. These findings suggest that in the injured mucosa, the pro-inflammatory milieu containing TNFalpha and PAF sets the stage for reparative events mediated by PAFR signaling.
Cell stem cell 2019 dec
Hic1 Defines Quiescent Mesenchymal Progenitor Subpopulations with Distinct Functions and Fates in Skeletal Muscle Regeneration.
Abstract
Abstract
Many adult tissues contain resident stem cells, such as the Pax7+ satellite cells within skeletal muscle, that regenerate parenchymal elements following damage. Tissue-resident mesenchymal progenitors (MPs) also participate in regeneration, although their function and fate in this process are unclear. Here, we identify Hypermethylated in cancer 1 (Hic1) as a marker of MPs in skeletal muscle and further show that Hic1 deletion leads to MP hyperplasia. Single-cell RNA-seq and ATAC-seq analysis of Hic1+ MPs in skeletal muscle shows multiple subpopulations, which we further show have distinct functions and lineage potential. Hic1+ MPs orchestrate multiple aspects of skeletal muscle regeneration by providing stage-specific immunomodulation and trophic and mechanical support. During muscle regeneration, Hic1+ derivatives directly contribute to several mesenchymal compartments including Col22a1-expressing cells within the myotendinous junction. Collectively, these findings demonstrate that HIC1 regulates MP quiescence and identifies MP subpopulations with transient and enduring roles in muscle regeneration.
Scientific reports 2018 NOV
Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic Cell Proliferation.
Abstract
Abstract
Chronic periodontitis (CP) is a microbial dysbiotic disease linked to increased risk of oral squamous cell carcinomas (OSCCs). To address the underlying mechanisms, mouse and human cell infection models and human biopsy samples were employed. We show that the 'keystone' pathogen Porphyromonas gingivalis, disrupts immune surveillance by generating myeloid-derived dendritic suppressor cells (MDDSCs) from monocytes. MDDSCs inhibit CTLs and induce FOXP3 + Tregs through an anti-apoptotic pathway. This pathway, involving pAKT1, pFOXO1, FOXP3, IDO1 and BIM, is activated in humans with CP and in mice orally infected with Mfa1 expressing P. gingivalis strains. Mechanistically, activation of this pathway, demonstrating FOXP3 as a direct FOXO1-target gene, was demonstrated by ChIP-assay in human CP gingiva. Expression of oncogenic but not tumor suppressor markers is consistent with tumor cell proliferation demonstrated in OSCC-P. gingivalis cocultures. Importantly, FimA + P. gingivalis strain MFI invades OSCCs, inducing inflammatory/angiogenic/oncogenic proteins stimulating OSCCs proliferation through CXCR4. Inhibition of CXCR4 abolished Pg-MFI-induced OSCCs proliferation and reduced expression of oncogenic proteins SDF-1/CXCR4, plus pAKT1-pFOXO1. Conclusively, P. gingivalis, through Mfa1 and FimA fimbriae, promotes immunosuppression and oncogenic cell proliferation, respectively, through a two-hit receptor-ligand process involving DC-SIGN+hi/CXCR4+hi, activating a pAKT+hipFOXO1+hiBIM-lowFOXP3+hi and IDO+hi- driven pathway, likely to impact the prognosis of oral cancers in patients with periodontitis.