EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion
Immunomagnetic negative selection cell isolation kit
概要
The EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion is designed to isolate monocytes and CD14+CD16+ monocytes from fresh or previously frozen peripheral blood mononuclear cells by negative selection. The CD14+CD16+ subset of monocytes (~10% in blood of healthy individuals) has characteristics of tissue macrophages and expands greatly in acute and chronic inflammatory disease. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-monocyte cells and magnetic particles. The cocktail also contains an antibody to human Fc receptor to minimize nonspecific binding. The labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube. For applications in which removal of all CD16+ cells is desired, we recommend the EasySep™ Human Monocyte Enrichment Kit (Catalog #19359), which contains anti-CD16.
Advantages
• Fast, easy-to-use and column-free
• Up to 81% purity
• Untouched, viable cells
• Up to 81% purity
• Untouched, viable cells
Components
- EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion (Catalog #19058)
- EasySep™ Human Monocyte Enrichment Cocktail without CD16 Depletion, 1 mL
- EasySep™ Magnetic Particles, 1 mL
- RoboSep™ Human Monocyte Enrichment Kit without CD16 Depletion with Filter Tips (Catalog #19058RF)
- EasySep™ Human Monocyte Enrichment Cocktail without CD16 Depletion, 1 mL
- EasySep™ Magnetic Particles, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Monocytes
Species
Human
Sample Source
PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion | 19058 | All | English |
| Product Information Sheet | RoboSep™ Human Monocyte Enrichment Kit without CD16 Depletion with Filter Tips | 19058RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion | 19058 | All | English |
| Safety Data Sheet 2 | EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion | 19058 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human Monocyte Enrichment Kit without CD16 Depletion with Filter Tips | 19058RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human Monocyte Enrichment Kit without CD16 Depletion with Filter Tips | 19058RF | All | English |
数据及文献
Data
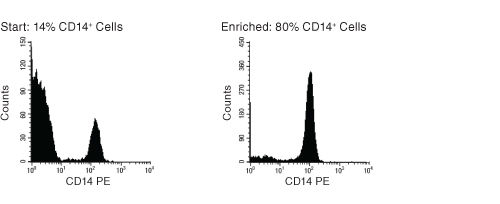
Figure 1. FACS Histogram Results Using EasySep™ Human Monocyte Enrichment Kit Without CD16 Depletion
Starting with freshly prepared peripheral blood mononuclear cells, the CD14+ cell content of the enriched fraction typically ranges from 73% - 81%. Slightly lower CD14+ cell purities may be obtained from samples that contain a large number of CD16+ cells.
Publications (18)
Journal of clinical medicine 2020 may
Inflammation-Induced Mucosal KYNU Expression Identifies Human Ileal Crohn's Disease.
Abstract
Abstract
The widely varying therapeutic response of patients with inflammatory bowel disease (IBD) continues to raise questions regarding the unclarified heterogeneity of pathological mechanisms promoting disease progression. While biomarkers for the differentiation of Crohn's disease (CD) versus ulcerative colitis (UC) have been suggested, specific markers for a CD subclassification in ileal CD versus colonic CD are still rare. Since an altered signature of the tryptophan metabolism is associated with chronic inflammatory disease, we sought to characterize potential biomarkers by focusing on the downstream enzymes and metabolites of kynurenine metabolism. Using immunohistochemical stainings, we analyzed and compared the mucosal tryptophan immune metabolism in bioptic samples from patients with active inflammation due to UC or CD versus healthy controls. Localization-specific quantification of immune cell infiltration, tryptophan-metabolizing enzyme expression and mucosal tryptophan downstream metabolite levels was performed. We found generally increased immune cell infiltrates in the tissue of all patients with IBD. However, in patients with CD, significant differences were found between regulatory T cell and neutrophil granulocyte infiltration in the ileum compared with the colon. Furthermore, we observed decreased kynurenine levels as well as strong kynureninase (KYNU) expression specifically in patients with ileal CD. Correspondingly, significantly elevated levels of the kynurenine metabolite 3-hydroxyanthranilic acid were detected in the ileal CD samples. Highlighting the heterogeneity of the different phenotypes of CD, we identified KYNU as a potential mucosal biomarker allowing the localization-specific differentiation of ileal CD versus colonic CD.
Cell systems 2020 mar
Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids.
Abstract
Abstract
Although the association between the microbiome and IBD and liver diseases is known, the cause and effect remain elusive. By connecting human microphysiological systems of the gut, liver, and circulating Treg and Th17 cells, we created a multi-organ model of ulcerative colitis (UC) ex vivo. The approach shows microbiome-derived short-chain fatty acids (SCFAs) to either improve or worsen UC severity, depending on the involvement of effector CD4 T cells. Using multiomics, we found SCFAs increased production of ketone bodies, glycolysis, and lipogenesis, while markedly reducing innate immune activation of the UC gut. However, during acute T cell-mediated inflammation, SCFAs exacerbated CD4+ T cell-effector function, partially through metabolic reprograming, leading to gut barrier disruption and hepatic injury. These paradoxical findings underscore the emerging utility of human physiomimetic technology in combination with systems immunology to study causality and the fundamental entanglement of immunity, metabolism, and tissue homeostasis.
Frontiers in immunology 2020
S100A12 Expression Is Modulated During Monocyte Differentiation and Reflects Periodontitis Severity.
Abstract
Abstract
S100A12 is a calcium-binding protein of the S100 subfamily of myeloid-related proteins that acts as an alarmin to induce a pro-inflammatory innate immune response. It has been linked to several chronic inflammatory diseases, however its role in the common oral immunopathology periodontitis is largely unknown. Previous in vitro monoculture experiments indicate that S100A12 production decreases during monocyte differentiation stages, while the regulation within tissue is poorly defined. This study evaluated S100A12 expression in monocyte subsets, during monocyte-to-macrophage differentiation and following polarization, both in monoculture and in a tissue context, utilizing a three-dimensional co-culture oral tissue model. Further, we explored the involvement of S100A12 in periodontitis by analyzing its expression in peripheral circulation and gingival tissue, as well as in saliva. We found that S100A12 expression was higher in classical than in non-classical monocytes. S100A12 expression and protein secretion declined significantly during monocyte-to-macrophage differentiation, while polarization of monocyte-derived macrophages had no effect on either. Peripheral monocytes from periodontitis patients had higher S100A12 expression than monocytes from controls, a difference particularly observed in the intermediate and non-classical monocyte subsets. Further, monocytes from periodontitis patients displayed an increased secretion of S100A12 compared with monocytes from controls. In oral tissue cultures, monocyte differentiation resulted in increased S100A12 secretion over time, which further increased after inflammatory stimuli. Likewise, S100A12 expression was higher in gingival tissue from periodontitis patients where monocyte-derived cells exhibited higher expression of S100A12 in comparison to non-periodontitis tissue. In line with our findings, patients with severe periodontitis had significantly higher levels of S100A12 in saliva compared to non-periodontitis patients, and the levels correlated to clinical periodontal parameters. Taken together, S100A12 is predominantly secreted by monocytes rather than by monocyte-derived cells. Moreover, S100A12 is increased in inflamed tissue cultures, potentially as a result of enhanced production by monocyte-derived cells. This study implicates the involvement of S100A12 in periodontitis pathogenesis, as evidenced by increased S100A12 expression in inflamed gingival tissue, which may be due to altered circulatory monocytes in periodontitis.
Cell host {\&} microbe 2020
Non-neutralizing Antibodies from a Marburg Infection Survivor Mediate Protection by Fc-Effector Functions and by Enhancing Efficacy of Other Antibodies.
Abstract
Abstract
Marburg virus (MARV) and Ebola virus (EBOV) belong to the family Filoviridae. MARV causes severe disease in humans with high fatality. We previously isolated a large panel of monoclonal antibodies (mAbs) from B cells of a human survivor with previous naturally acquired MARV infection. Here, we characterized functional properties of these mAbs and identified non-neutralizing mAbs targeting the glycoprotein (GP) 2 portion of the mucin-like domain (MLD) of MARV GP, termed the wing region. One mAb targeting the GP2 wing, MR228, showed therapeutic protection in mice and guinea pigs infected with MARV. The protection was mediated by the Fc fragment functions of MR228. Binding of another GP2 wing-specific non-neutralizing mAb, MR235, to MARV GP increased accessibility of epitopes in the receptor-binding site (RBS) for neutralizing mAbs, resulting in enhanced virus neutralization by these mAbs. These findings highlight an important role for non-neutralizing mAbs during natural human MARV infection.
Cellular molecular immunology 2019 mar
ACPAs promote IL-1beta production in rheumatoid arthritis by activating the NLRP3 inflammasome.
Abstract
Abstract
OBJECTIVES Anti-citrullinated protein antibodies (ACPAs) are a group of autoantibodies targeted against citrullinated proteins/peptides and are informative rheumatoid arthritis (RA) biomarkers. ACPAs also play a crucial role in RA pathogenesis, and their underlying mechanism merits investigation. METHODS Immunohistochemical (IHC) assays were carried out to determine IL-1beta levels in ACPA+ and ACPA- RA patients. PBMC-derived monocytes were differentiated into macrophages before stimulation with ACPAs purified from RA patients. The localization and interaction of molecules were analyzed by confocal microscopy, co-IP, and surface plasmon resonance. RESULTS In our study, we found that IL-1beta levels were elevated in ACPA+ RA patients and that ACPAs promoted IL-1beta production by PBMC-derived macrophages. ACPAs interacted with CD147 to enhance the interaction between CD147 and integrin beta1 and, in turn, activate the Akt/NF-kappaB signaling pathway. The nuclear localization of p65 promoted the expression of NLRP3 and pro-IL-1beta, resulting in priming. Moreover, ACPA stimulation activated pannexin channels, leading to ATP release. The accumulated ATP bound to the P2X7 receptor, leading to NLRP3 inflammasome activation. CONCLUSIONS Our study suggests a new hypothesis regarding IL-1beta production in RA involving ACPAs, which may be a potential therapeutic target in RA treatment.
Cell reports 2019 jan
High-Affinity Bent beta2-Integrin Molecules in Arresting Neutrophils Face Each Other through Binding to ICAMs In cis.
Abstract
Abstract
Leukocyte adhesion requires beta2-integrin activation. Resting integrins exist in a bent-closed conformation-i.e., not extended (E-) and not high affinity (H-)-unable to bind ligand. Fully activated E+H+ integrin binds intercellular adhesion molecules (ICAMs) expressed on the opposing cell in trans. E-H- transitions to E+H+ through E+H- or through E-H+, which binds to ICAMs on the same cell in cis. Spatial patterning of activated integrins is thought to be required for effective arrest, but no high-resolution cell surface localization maps of activated integrins exist. Here, we developed Super-STORM by combining super-resolution microscopy with molecular modeling to precisely localize activated integrin molecules and identify the molecular patterns of activated integrins on primary human neutrophils. At the time of neutrophil arrest, E-H+ integrins face each other to form oriented (non-random) nanoclusters. To address the mechanism causing this pattern, we blocked integrin binding to ICAMs in cis, which significantly relieved the face-to-face orientation.