EasySep™ Human CD138 Positive Selection Kit II
Immunomagnetic positive selection cell isolation kit
概要
The EasySep™ Human CD138 Positive Selection Kit II is designed to isolate CD138+ (syndecan-1) cells from fresh or previously frozen bone marrow or peripheral blood mononuclear cells by positive selection. Desired cells are targeted with Tetrameric Antibody Complexes recognizing CD138 and dextran-coated magnetic particles. This cocktail also contains an antibody to human Fc receptor to minimize nonspecific binding. Labeled cells are separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off. The CD138 antigen is expressed on normal and malignant plasma cells (but not mature B cells).
This product is an improved version of the EasySep™ Human CD138 Positive Selection Kit (Catalog #18357) that provides highly purified cells using a shorter protocol.
This product is an improved version of the EasySep™ Human CD138 Positive Selection Kit (Catalog #18357) that provides highly purified cells using a shorter protocol.
Advantages
• Fast and easy-to-use
• No columns required
• No columns required
Components
- EasySep™ Human CD138 Positive Selection Kit II (Catalog #17877)
- EasySep™ Human CD138 Positive Selection Kit II Cocktail, 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Human CD138 Positive Selection Kit II with Filter Tips (Catalog #17877RF)
- EasySep™ Human CD138 Positive Selection Kit II Cocktail, 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
B Cells, Plasma
Species
Human
Sample Source
Bone Marrow, PBMC
Selection Method
Positive
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Cancer Research, Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD138 Positive Selection Kit II | 17877 | All | English |
| Product Information Sheet | RoboSep™ Human CD138 Positive Selection Kit II | 17877RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD138 Positive Selection Kit II | 17877 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD138 Positive Selection Kit II | 17877 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD138 Positive Selection Kit II | 17877RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD138 Positive Selection Kit II | 17877RF | All | English |
数据及文献
Data
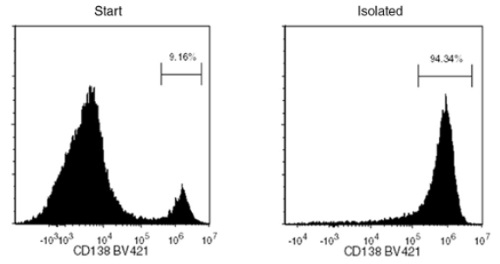
Starting with thawed PBMCs spiked with a multiple myeloma cell line, U266, the CD138+ cell content of the isolated fraction typically ranges from 93.0 - 98.2%. In the above example, the purities of the start and final isolated fractions are 9.16% and 94.34%, respectively.
Publications (7)
Scientific reports 2020 jan
Silencing of SENP2 in Multiple Myeloma Induces Bortezomib Resistance by Activating NF-$\kappa$B Through the Modulation of I$\kappa$B$\alpha$ Sumoylation.
Abstract
Abstract
The proteasome inhibitor bortezomib is the most successfully applied chemotherapeutic drug for treating multiple myeloma. However, its clinical efficacy reduced due to resistance development. The underlying molecular mechanisms of bortezomib resistance are poorly understood. In this study, by combining in silico analysis and sgRNA library based drug resistance screening assay, we identified SENP2 (Sentrin/SUMO-specific proteases-2) as a bortezomib sensitive gene and found its expression highly downregulated in bortezomib resistant multiple myeloma patient's samples. Furthermore, down regulation of SENP2 in multiple myeloma cell line RPMI8226 alleviated bortezomib induced cell proliferation inhibition and apoptosis, whereas, overexpression of SENP2 sensitized these cells to bortezomib treatment. We further demonstrate that knockdown of SENP2 in RPMI8226 cells increased SUMO2 conjugated I$\kappa$B$\alpha$ that resulted in the activation of NF-$\kappa$B. Taken together, we report that silencing of SENP2 and consequent activation of NF-$\kappa$B through the modulation of I$\kappa$B$\alpha$ sumoylation as a novel mechanism inducing bortezomib resistance in multiple myeloma.
Leukemia 2020 jan
BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8+ cytotoxic T lymphocytes against multiple myeloma: clinical applications.
Abstract
Abstract
The purpose of these studies was to develop and characterize B-cell maturation antigen (BCMA)-specific peptide-encapsulated nanoparticle formulations to efficiently evoke BCMA-specific CD8+ cytotoxic T lymphocytes (CTL) with poly-functional immune activities against multiple myeloma (MM). Heteroclitic BCMA72-80 [YLMFLLRKI] peptide-encapsulated liposome or poly(lactic-co-glycolic acid) (PLGA) nanoparticles displayed uniform size distribution and increased peptide delivery to human dendritic cells, which enhanced induction of BCMA-specific CTL. Distinct from liposome-based nanoparticles, PLGA-based nanoparticles demonstrated a gradual increase in peptide uptake by antigen-presenting cells, and induced BCMA-specific CTL with higher anti-tumor activities (CD107a degranulation, CTL proliferation, and IFN-$\gamma$/IL-2/TNF-$\alpha$ production) against primary CD138+ tumor cells and MM cell lines. The improved functional activities were associated with increased Tetramer+/CD45RO+ memory CTL, CD28 upregulation on Tetramer+ CTL, and longer maintenance of central memory (CCR7+ CD45RO+) CTL, with the highest anti-MM activity and less differentiation into effector memory (CCR7- CD45RO+) CTL. These results provide the framework for therapeutic application of PLGA-based BCMA immunogenic peptide delivery system, rather than free peptide, to enhance the induction of BCMA-specific CTL with poly-functional Th1-specific anti-MM activities. These results demonstrate the potential clinical utility of PLGA nanotechnology-based cancer vaccine to enhance BCMA-targeted immunotherapy against myeloma.
Leukemia 2020 apr
Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma.
Abstract
Abstract
Oncolytic virus therapy leads to immunogenic death of virus-infected tumor cells and this has been shown in preclinical models to enhance the cytotoxic T-lymphocyte response against tumor-associated antigens (TAAs), leading to killing of uninfected tumor cells. To investigate whether oncolytic virotherapy can increase immune responses to tumor antigens in human subjects, we studied T-cell responses against a panel of known myeloma TAAs using PBMC samples obtained from ten myeloma patients before and after systemic administration of an oncolytic measles virus encoding sodium iodide symporter (MV-NIS). Despite their prior exposures to multiple immunosuppressive antimyeloma treatment regimens, T-cell responses to some of the TAAs were detectable even before measles virotherapy. Measurable baseline T-cell responses against MAGE-C1 and hTERT were present. Furthermore, MV-NIS treatment significantly (P {\textless} 0.05) increased T-cell responses against MAGE-C1 and MAGE-A3. Interestingly, one patient who achieved complete remission after MV-NIS therapy had strong baseline T-cell responses both to measles virus proteins and to eight of the ten tested TAAs. Our data demonstrate that oncolytic virotherapy can function as an antigen agnostic vaccine, increasing cytotoxic T-lymphocyte responses against TAAs in patients with multiple myeloma, providing a basis for continued exploration of this modality in combination with immune checkpoint blockade.
Cell death discovery 2019
Prosurvival autophagy is regulated by protein kinase CK1 alpha in multiple myeloma.
Abstract
Abstract
Multiple myeloma (MM) is a tumor of plasma cells (PCs). Due to the intense immunoglobulin secretion, PCs are prone to endoplasmic reticulum stress and activate several stress-managing pathways, including autophagy. Indeed, autophagy deregulation is maladaptive for MM cells, resulting in cell death. CK1alpha, a pro-survival kinase in MM, has recently been involved as a regulator of the autophagic flux and of the transcriptional competence of the autophagy-related transcription factor FOXO3a in several cancers. In this study, we investigated the role of CK1alpha in autophagy in MM. To study the autophagic flux we generated clones of MM cell lines expressing the mCherry-eGFP-LC3B fusion protein. We observed that CK1 inhibition with the chemical ATP-competitive CK1 alpha/delta inhibitor D4476 resulted in an impaired autophagic flux, likely due to an alteration of lysosomes acidification. However, D4476 caused the accumulation of the transcription factor FOXO3a in the nucleus, and this was paralleled by the upregulation of mRNA coding for autophagic genes. Surprisingly, silencing of CK1alpha by RNA interference triggered the autophagic flux. However, FOXO3a did not shuttle into the nucleus and the transcription of autophagy-related FOXO3a-dependent genes was not observed. Thus, while the chemical inhibition with the dual CK1alpha/delta inhibitor D4476 induced cell death as a consequence of an accumulation of ineffective autophagic vesicles, on the opposite, CK1alpha silencing, although it also determined apoptosis, triggered a full activation of the early autophagic flux, which was then not supported by the upregulation of autophagic genes. Taken together, our results indicate that the family of CK1 kinases may profoundly influence MM cells survival also through the modulation of the autophagic pathway.
Blood advances 2018 OCT
An inhibitor of proteasome $\beta$2 sites sensitizes myeloma cells to immunoproteasome inhibitors.
Abstract
Abstract
Proteasome inhibitors bortezomib, carfilzomib and ixazomib (approved by the US Food and Drug Administration [FDA]) induce remissions in patients with multiple myeloma (MM), but most patients eventually become resistant. MM and other hematologic malignancies express ubiquitous constitutive proteasomes and lymphoid tissue-specific immunoproteasomes; immunoproteasome expression is increased in resistant patients. Immunoproteasomes contain 3 distinct pairs of active sites, $\beta$5i, $\beta$1i, and $\beta$2i, which are different from their constitutive $\beta$5c, $\beta$1c, and $\beta$2c counterparts. Bortezomib and carfilzomib block $\beta$5c and $\beta$5i sites. We report here that pharmacologically relevant concentrations of $\beta$5i-specific inhibitor ONX-0914 show cytotoxicity in MM cell lines similar to that of carfilzomib and bortezomib. In addition, increasing immunoproteasome expression by interferon-$\gamma$ increases sensitivity to ONX-0914 but not to carfilzomib. LU-102, an inhibitor of $\beta$2 sites, dramatically sensitizes MM cell lines and primary cells to ONX-0914. ONX-0914 synergizes with all FDA-approved proteasome inhibitors in MM in vitro and in vivo. Thus, immunoproteasome inhibitors, currently in clinical trials for the treatment of autoimmune diseases, should also be considered for the treatment of MM.
Cancer genetics 2018 DEC
Assessing genome-wide copy number aberrations and copy-neutral loss-of-heterozygosity as best practice: An evidence-based review from the Cancer Genomics Consortium working group for plasma cell disorders.
Abstract
Abstract
BACKGROUND Plasma cell neoplasms (PCNs) encompass a spectrum of disorders including monoclonal gammopathy of undetermined significance, smoldering myeloma, plasma cell myeloma, and plasma cell leukemia. Molecular subtypes have been defined by recurrent cytogenetic abnormalities and somatic mutations that are prognostic and predictive. Karyotype and fluorescence in situ hybridization (FISH) have historically been used to guide management; however, new technologies and markers raise the need to reassess current testing algorithms. METHODS We convened a panel of representatives from international clinical laboratories to capture current state-of-the-art testing from published reports and to put forward recommendations for cytogenomic testing of plasma cell neoplasms. We reviewed 65 papers applying FISH, chromosomal microarray (CMA), next-generation sequencing, and gene expression profiling for plasma cell neoplasm diagnosis and prognosis. We also performed a survey of our peers to capture current laboratory practice employed outside our working group. RESULTS Plasma cell enrichment is widely used prior to FISH testing, most commonly by magnetic bead selection. A variety of strategies for direct, short- and long-term cell culture are employed to ensure clonal representation for karyotyping. Testing of clinically-informative 1p/1q, del(13q) and del(17p) are common using karyotype, FISH and, increasingly, CMA testing. FISH for a variety of clinically-informative balanced IGH rearrangements is prevalent. Literature review found that CMA analysis can detect abnormalities in 85-100{\%} of patients with PCNs; more specifically, in 5-53{\%} (median 14{\%}) of cases otherwise normal by FISH and cytogenetics. CMA results in plasma cell neoplasms are usually complex, with alteration counts ranging from 1 to 74 (median 10-20), primarily affecting loci not covered by FISH testing. Emerging biomarkers include structural alterations of MYC as well as somatic mutations of KRAS, NRAS, BRAF, and TP53. Together, these may be measured in a comprehensive manner by a combination of newer technologies including CMA and next-generation sequencing (NGS). Our survey suggests most laboratories have, or are soon to have, clinical CMA platforms, with a desire to move to NGS assays in the future. CONCLUSION We present an overview of current practices in plasma cell neoplasm testing as well as an algorithm for integrated FISH and CMA testing to guide treatment of this disease.