EasySep™ Human CD34 Positive Selection Kit II
Immunomagnetic positive selection kit
概要
The EasySep™ Human CD34 Positive Selection Kit II is designed to isolate CD34+ cells from fresh or previously frozen bone marrow or mobilized peripheral blood mononuclear cells, or from previously frozen cord blood mononuclear cells, or from hESC/hiPSC-derived hematopoietic cells by positive selection. Desired cells are targeted with Tetrameric Antibody Complexes recognizing CD34 and dextran-coated magnetic particles. The cocktail also contains an antibody to human Fc receptor to minimize nonspecific binding. Labeled cells are separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off. The CD34 antigen is expressed on hematopoietic stem and progenitor cells.
This product can be used in place of the EasySep™ Human CD34 Positive Selection Kit (Catalog #18056) for even faster cell isolations.
This product can be used in place of the EasySep™ Human CD34 Positive Selection Kit (Catalog #18056) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 99% purity
• No columns required
• Up to 99% purity
• No columns required
Components
- EasySep™ Human CD34 Positive Selection Kit II (Catalog #17856)
- EasySep™ Human CD34 Positive Selection Cocktail, 1 x 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 x 1 mL
- RoboSep™ Human CD34 Positive Selection Kit II with Filter Tips (Catalog #17856RF)
- EasySep™ Human CD34 Positive Selection Cocktail, 1 x 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 x 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Hematopoietic Stem and Progenitor Cells, Pluripotent Stem Cells
Species
Human
Sample Source
Bone Marrow, Cord Blood, Pluripotent Stem Cells, Other, PBMC, Whole Blood
Selection Method
Positive
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Chimerism, Immunology, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD34 Positive Selection Kit II | 17856, 17856RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD34 Positive Selection Kit II | 17856 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD34 Positive Selection Kit II | 17856, 17856RF | All | English |
| Safety Data Sheet 3 | EasySep™ Human CD34 Positive Selection Kit II | 17856, 17856RF | All | English |
数据及文献
Data
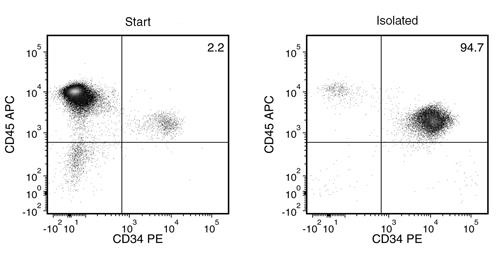
Starting with cord blood, mobilized peripheral blood or bone marrow MNCs, or ES and iPS cell cultures, the CD34+ cell content of the isolated fraction is typically 93.5 ± 1.1% (mean ± SD using the purple EasySep™ Magnet). In the above example using frozen cord blood, the purities of the start and final isolated fractions are 2.2% and 94.7%, respectively.
Publications (4)
Clinical cancer research : an official journal of the American Association for Cancer Research 2019 jul
Combined CD28 and 4-1BB Costimulation Potentiates Affinity-tuned Chimeric Antigen Receptor-engineered T Cells.
Abstract
Abstract
PURPOSE Targeting nonspecific, tumor-associated antigens (TAA) with chimeric antigen receptors (CAR) requires specific attention to restrict possible detrimental on-target/off-tumor effects. A reduced affinity may direct CAR-engineered T (CAR-T) cells to tumor cells expressing high TAA levels while sparing low expressing normal tissues. However, decreasing the affinity of the CAR-target binding may compromise the overall antitumor effects. Here, we demonstrate the prime importance of the type of intracellular signaling on the function of low-affinity CAR-T cells. EXPERIMENTAL DESIGN We used a series of single-chain variable fragments (scFv) with five different affinities targeting the same epitope of the multiple myeloma-associated CD38 antigen. The scFvs were incorporated in three different CAR costimulation designs and we evaluated the antitumor functionality and off-tumor toxicity of the generated CAR-T cells in vitro and in vivo. RESULTS We show that the inferior cytotoxicity and cytokine secretion mediated by CD38 CARs of very low-affinity (Kd {\textless} 1.9 × 10-6 mol/L) bearing a 4-1BB intracellular domain can be significantly improved when a CD28 costimulatory domain is used. Additional 4-1BB signaling mediated by the coexpression of 4-1BBL provided the CD28-based CD38 CAR-T cells with superior proliferative capacity, preservation of a central memory phenotype, and significantly improved in vivo antitumor function, while preserving their ability to discriminate target antigen density. CONCLUSIONS A combinatorial costimulatory design allows the use of very low-affinity binding domains (Kd {\textless} 1 mumol/L) for the construction of safe but also optimally effective CAR-T cells. Thus, very-low-affinity scFvs empowered by selected costimulatory elements can enhance the clinical potential of TAA-targeting CARs.
Journal of immunology (Baltimore, Md. : 1950) 2019 feb
Induction and Therapeutic Targeting of Human NPM1c+ Myeloid Leukemia in the Presence of Autologous Immune System in Mice.
Abstract
Abstract
Development of targeted cancer therapy requires a thorough understanding of mechanisms of tumorigenesis as well as mechanisms of action of therapeutics. This is challenging because by the time patients are diagnosed with cancer, early events of tumorigenesis have already taken place. Similarly, development of cancer immunotherapies is hampered by a lack of appropriate small animal models with autologous human tumor and immune system. In this article, we report the development of a mouse model of human acute myeloid leukemia (AML) with autologous immune system for studying early events of human leukemogenesis and testing the efficacy of immunotherapeutics. To develop such a model, human hematopoietic stem/progenitor cells (HSPC) are transduced with lentiviruses expressing a mutated form of nucleophosmin (NPM1), referred to as NPM1c. Following engraftment into immunodeficient mice, transduced HSPCs give rise to human myeloid leukemia, whereas untransduced HSPCs give rise to human immune cells in the same mice. The de novo AML, with CD123+ leukemic stem or initiating cells (LSC), resembles NPM1c+ AML from patients. Transcriptional analysis of LSC and leukemic cells confirms similarity of the de novo leukemia generated in mice with patient leukemia and suggests Myc as a co-operating factor in NPM1c-driven leukemogenesis. We show that a bispecific conjugate that binds both CD3 and CD123 eliminates CD123+ LSCs in a T cell-dependent manner both in vivo and in vitro. These results demonstrate the utility of the NPM1c+ AML model with an autologous immune system for studying early events of human leukemogenesis and for evaluating efficacy and mechanism of immunotherapeutics.
Cancer cell 2019 aug
Mechanisms of Progression of Myeloid Preleukemia to Transformed Myeloid Leukemia in Children with Down Syndrome.
Abstract
Abstract
Myeloid leukemia in Down syndrome (ML-DS) clonally evolves from transient abnormal myelopoiesis (TAM), a preleukemic condition in DS newborns. To define mechanisms of leukemic transformation, we combined exome and targeted resequencing of 111 TAM and 141 ML-DS samples with functional analyses. TAM requires trisomy 21 and truncating mutations in GATA1; additional TAM variants are usually not pathogenic. By contrast, in ML-DS, clonal and subclonal variants are functionally required. We identified a recurrent and oncogenic hotspot gain-of-function mutation in myeloid cytokine receptor CSF2RB. By a multiplex CRISPR/Cas9 screen in an in vivo murine TAM model, we tested loss-of-function of 22 recurrently mutated ML-DS genes. Loss of 18 different genes produced leukemias that phenotypically, genetically, and transcriptionally mirrored ML-DS.
Acta neuropathologica communications 2017 dec
CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer's PSEN2 N141I neurons.
Abstract
Abstract
Basal forebrain cholinergic neurons (BFCNs) are believed to be one of the first cell types to be affected in all forms of AD, and their dysfunction is clinically correlated with impaired short-term memory formation and retrieval. We present an optimized in vitro protocol to generate human BFCNs from iPSCs, using cell lines from presenilin 2 (PSEN2) mutation carriers and controls. As expected, cell lines harboring the PSEN2 N141I mutation displayed an increase in the A$\beta$42/40 in iPSC-derived BFCNs. Neurons derived from PSEN2 N141I lines generated fewer maximum number of spikes in response to a square depolarizing current injection. The height of the first action potential at rheobase current injection was also significantly decreased in PSEN2 N141I BFCNs. CRISPR/Cas9 correction of the PSEN2 point mutation abolished the electrophysiological deficit, restoring both the maximal number of spikes and spike height to the levels recorded in controls. Increased A$\beta$42/40 was also normalized following CRISPR/Cas-mediated correction of the PSEN2 N141I mutation. The genome editing data confirms the robust consistency of mutation-related changes in A$\beta$42/40 ratio while also showing a PSEN2-mutation-related alteration in electrophysiology.