EasySep™ Human CD8+ T Cell Isolation Kit
8-Minute cell isolation kit using immunomagnetic negative selection
概要
The EasySep™ Human CD8+ T Cell Isolation Kit is designed to isolate CD8+ T cells from fresh or previously frozen peripheral blood mononuclear cells or washed leukapheresis samples by immunomagnetic negative selection. The EasySep™ procedure involves labeling unwanted cells with antibody complexes and magnetic particles. The magnetically labeled cells are separated from the untouched desired cells by using an EasySep™ magnet and simply pouring or pipetting the desired cells into a new tube.
This product can be used in place of the EasySep™ Human CD8+ T Cell Enrichment Kit (Catalog #19053) for even faster cell isolations.
This product can be used in place of the EasySep™ Human CD8+ T Cell Enrichment Kit (Catalog #19053) for even faster cell isolations.
Advantages
• Fast, easy-to-use and column-free
• Up to 91% purity with high recovery
• Untouched, viable cells
• Up to 91% purity with high recovery
• Untouched, viable cells
Components
- EasySep™ Human CD8+ T Cell Isolation Kit (Catalog #17953)
- EasySep™ Human CD8+ T Cell Isolation Cocktail, 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Human CD8+ T Cell Isolation Kit (Catalog #17953RF)
- EasySep™ Human CD8+ T Cell Isolation Cocktail, 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD8+
Species
Human
Sample Source
Leukapheresis, PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD8+ T Cell Isolation Kit | 17953 | All | English |
| Product Information Sheet | RoboSep™ Human CD8+ T Cell Isolation Kit | 17953RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD8+ T Cell Isolation Kit | 17953 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD8+ T Cell Isolation Kit | 17953 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD8+ T Cell Isolation Kit | 17953RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD8+ T Cell Isolation Kit | 17953RF | All | English |
数据及文献
Data
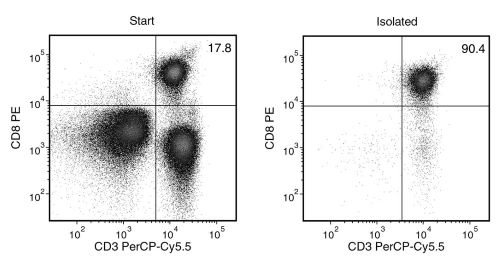
Figure 1. EasySep™ Human CD8+ T Cell Isolation Kit
Starting with human peripheral blood mononuclear cells (PBMCs), the CD8+ T cell content (CD3+CD8+) of the isolated fraction is typically 85.6 ± 4.9% (mean ± SD for the purple EasySep™ Magnet).
Publications (10)
Nature 2019 may
CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy.
Abstract
Abstract
Cancer immunotherapy restores or enhances the effector function of CD8+ T cells in the tumour microenvironment1,2. CD8+ T cells activated by cancer immunotherapy clear tumours mainly by inducing cell death through perforin-granzyme and Fas-Fas ligand pathways3,4. Ferroptosis is a form of cell death that differs from apoptosis and results from iron-dependent accumulation of lipid peroxide5,6. Although it has been investigated in vitro7,8, there is emerging evidence that ferroptosis might be implicated in a variety of pathological scenarios9,10. It is unclear whether, and how, ferroptosis is involved in T cell immunity and cancer immunotherapy. Here we show that immunotherapy-activated CD8+ T cells enhance ferroptosis-specific lipid peroxidation in tumour cells, and that increased ferroptosis contributes to the anti-tumour efficacy of immunotherapy. Mechanistically, interferon gamma (IFNgamma) released from CD8+ T cells downregulates the expression of SLC3A2 and SLC7A11, two subunits of the glutamate-cystine antiporter system xc-, impairs the uptake of cystine by tumour cells, and as a consequence, promotes tumour cell lipid peroxidation and ferroptosis. In mouse models, depletion of cystine or cysteine by cyst(e)inase (an engineered enzyme that degrades both cystine and cysteine) in combination with checkpoint blockade synergistically enhanced T cell-mediated anti-tumour immunity and induced ferroptosis in tumour cells. Expression of system xc- was negatively associated, in cancer patients, with CD8+ T cell signature, IFNgamma expression, and patient outcome. Analyses of human transcriptomes before and during nivolumab therapy revealed that clinical benefits correlate with reduced expression of SLC3A2 and increased IFNgamma and CD8. Thus, T cell-promoted tumour ferroptosis is an anti-tumour mechanism, and targeting this pathway in combination with checkpoint blockade is a potential therapeutic approach.
Nature metabolism 2019 jul
Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection.
Abstract
Abstract
Spontaneous control of human immunodeficiency virus (HIV) is generally associated with an enhanced capacity of CD8+ T cells to eliminate infected CD4+ T cells, but the molecular characteristics of these highly functional CD8+ T cells are largely unknown. In the present study, using single-cell analysis, it was shown that HIV-specific, central memory CD8+ T cells from spontaneous HIV controllers (HICs) and antiretrovirally treated non-controllers have opposing transcriptomic profiles. Genes linked to effector functions and survival are upregulated in cells from HICs. In contrast, genes associated with activation, exhaustion and glycolysis are upregulated in cells from non-controllers. It was shown that HIV-specific CD8+ T cells from non-controllers are largely glucose dependent, whereas those from HICs have more diverse metabolic resources that enhance both their survival potential and their capacity to develop anti-HIV effector functions. The functional efficiency of the HIV-specific CD8+ T cell response in HICs is thus engraved in their memory population and related to their metabolic programme. Metabolic reprogramming in vitro through interleukin-15 treatment abrogated the glucose dependency and enhanced the antiviral potency of HIV-specific CD8+ T cells from non-controllers.
Cell and tissue research 2019 dec
Characterizing the effects of hypoxia on the metabolic profiles of mesenchymal stromal cells derived from three tissue sources using chemical isotope labeling liquid chromatography-mass spectrometry.
Abstract
Abstract
Microenvironmental factors such as oxygen concentration mediate key effects on the biology of mesenchymal stromal cells (MSCs). Herein, we performed an in-depth characterization of the metabolic behavior of MSCs derived from the placenta, umbilical cord, and adipose tissue (termed hPMSCs, UC-MSCs, and AD-MSCs, respectively) at physiological (hypoxic; 5{\%} oxygen [O2]) and standardized (normoxic; 21{\%} O2) O2 concentrations using chemical isotope labeling liquid chromatography-mass spectrometry. 12C- and 13C-isotope dansylation (Dns) labeling was used to analyze the amine/phenol submetabolome, and 2574 peak pairs or metabolites were detected and quantified, from which 52 metabolites were positively identified using a library of 275 Dns-metabolite standards; 2189 metabolites were putatively identified. Next, we identified six metabolites using the Dns library, as well as 14 hypoxic biomarkers from the human metabolome database out of 96 altered metabolites. Ultimately, metabolic pathway analyses were performed to evaluate the associated pathways. Based on pathways identified using the Kyoto Encyclopedia of Genes and Genomes, we identified significant changes in the metabolic profiles of MSCs in response to different O2 concentrations. These results collectively suggest that O2 concentration has the strongest influence on hPMSCs metabolic characteristics, and that 5{\%} O2 promotes arginine and proline metabolism in hPMSCs and UC-MSCs but decreases gluconeogenesis (alanine-glucose) rates in hPMSCs and AD-MSCs. These changes indicate that MSCs derived from different sources exhibit distinct metabolic profiles.
Cancer immunology research 2019
Siglec-9 Regulates an Effector Memory CD8+ T-cell Subset That Congregates in the Melanoma Tumor Microenvironment.
Abstract
Abstract
Emerging evidence suggests an immunosuppressive role of altered tumor glycosylation due to downregulation of innate immune responses via immunoregulatory Siglecs. In contrast, human T cells, a major anticancer effector cell, only rarely express Siglecs. However, here, we report that the majority of intratumoral, but not peripheral blood, cytotoxic CD8+ T cells expressed Siglec-9 in melanoma. We identified Siglec-9+ CD8+ T cells as a subset of effector memory cells with high functional capacity and signatures of clonal expansion. This cytotoxic T-cell subset was functionally inhibited in the presence of Siglec-9 ligands or by Siglec-9 engagement by specific antibodies. TCR signaling pathways and key effector functions (cytotoxicity, cytokine production) of CD8+ T cells were suppressed by Siglec-9 engagement, which was associated with the phosphorylation of the inhibitory protein tyrosine phosphatase SHP-1, but not SHP-2. Expression of cognate Siglec-9 ligands was observed on the majority of tumor cells in primary and metastatic melanoma specimens. Targeting the tumor-restricted, glycosylation-dependent Siglec-9 axis may unleash this intratumoral T-cell subset, while confining T-cell activation to the tumor microenvironment.
Science signaling 2018 AUG
Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function.
Abstract
Abstract
Chimeric antigen receptors (CARs) link an antigen recognition domain to intracellular signaling domains to redirect T cell specificity and function. T cells expressing CARs with CD28/CD3$\zeta$ or 4-1BB/CD3$\zeta$ signaling domains are effective at treating refractory B cell malignancies but exhibit differences in effector function, clinical efficacy, and toxicity that are assumed to result from the activation of divergent signaling cascades. We analyzed stimulation-induced phosphorylation events in primary human CD8+ CD28/CD3$\zeta$ and 4-1BB/CD3$\zeta$ CAR T cells by mass spectrometry and found that both CAR constructs activated similar signaling intermediates. Stimulation of CD28/CD3$\zeta$ CARs activated faster and larger-magnitude changes in protein phosphorylation, which correlated with an effector T cell-like phenotype and function. In contrast, 4-1BB/CD3$\zeta$ CAR T cells preferentially expressed T cell memory-associated genes and exhibited sustained antitumor activity against established tumors in vivo. Mutagenesis of the CAR CD28 signaling domain demonstrated that the increased CD28/CD3$\zeta$ CAR signal intensity was partly related to constitutive association of Lck with this domain in CAR complexes. Our data show that CAR signaling pathways cannot be predicted solely by the domains used to construct the receptor and that signal strength is a key determinant of T cell fate. Thus, tailoring CAR design based on signal strength may lead to improved clinical efficacy and reduced toxicity.
Cell 2016 MAY
Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer.
Abstract
Abstract
Effector T cells and fibroblasts are major components in the tumor microenvironment. The means through which these cellular interactions affect chemoresistance is unclear. Here, we show that fibroblasts diminish nuclear accumulation of platinum in ovarian cancer cells, resulting in resistance to platinum-based chemotherapy. We demonstrate that glutathione and cysteine released by fibroblasts contribute to this resistance. CD8(+) T cells abolish the resistance by altering glutathione and cystine metabolism in fibroblasts. CD8(+) T-cell-derived interferon (IFN)γ controls fibroblast glutathione and cysteine through upregulation of gamma-glutamyltransferases and transcriptional repression of system xc(-) cystine and glutamate antiporter via the JAK/STAT1 pathway. The presence of stromal fibroblasts and CD8(+) T cells is negatively and positively associated with ovarian cancer patient survival, respectively. Thus, our work uncovers a mode of action for effector T cells: they abrogate stromal-mediated chemoresistance. Capitalizing upon the interplay between chemotherapy and immunotherapy holds high potential for cancer treatment.