EasySep™ Human CD8+ T Cell Enrichment Kit
Immunomagnetic negative selection kit
概要
The EasySep™ Human CD8+ T Cell Enrichment Kit is designed to isolate CD8+ T cells from fresh or previously frozen peripheral blood mononuclear cells or ammonium chloride-lysed leukapheresis sample by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-CD8+ T cells and glycophorin A and dextran-coated magnetic particles. The labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
For even faster cell isolations, we recommend the new EasySep™ Human CD8+ T Cell Isolation Kit (17953) which isolates cells in just 8 minutes.
For even faster cell isolations, we recommend the new EasySep™ Human CD8+ T Cell Isolation Kit (17953) which isolates cells in just 8 minutes.
Advantages
• Fast, easy-to-use and column-free
• Up to 95% purity
• Untouched, viable cells
• Up to 95% purity
• Untouched, viable cells
Components
- EasySep™ Human CD8+ T Cell Enrichment Kit (Catalog #19053)
- EasySep™ Human CD8 T Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 3 x 1 mL
- RoboSep™ Human CD8+ T Cell Enrichment Kit with Filter Tips (Catalog #19053RF)
- EasySep™ Human CD8 T Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 3 x 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD8+
Species
Human
Sample Source
Leukapheresis, PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD8+ T Cell Enrichment Kit | 19053 | All | English |
| Product Information Sheet | RoboSep™ Human CD8+ T Cell Enrichment Kit with Filter Tips | 19053RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD8+ T Cell Enrichment Kit | 19053 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD8+ T Cell Enrichment Kit | 19053 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD8+ T Cell Enrichment Kit with Filter Tips | 19053RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD8+ T Cell Enrichment Kit with Filter Tips | 19053RF | All | English |
数据及文献
Data
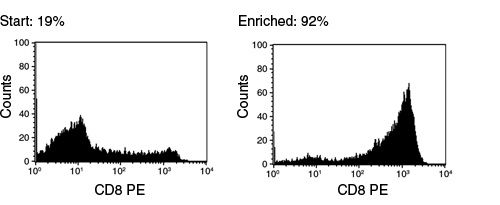
Figure 1. FACS Histogram Results Using EasySep™ Human CD8+ T Cell Enrichment Kit
Starting with frozen mononuclear cells, the CD8+ cell content of the enriched fraction typically ranges from 84 - 95%
Publications (12)
Frontiers in pharmacology 2020
A Compartmentalized Reduction in Membrane-Proximal Calmodulin Reduces the Immune Surveillance Capabilities of CD8+ T Cells in Head and Neck Cancer.
Abstract
Abstract
The limited ability of cytotoxic CD8+ T cells to infiltrate solid tumors and function within the tumor microenvironment presents a major roadblock to effective immunotherapy. Ion channels and Ca2+-dependent signaling events control the activity of T cells and are implicated in the failure of immune surveillance in cancer. Reduced KCa3.1 channel activity mediates the heightened inhibitory effect of adenosine on the chemotaxis of circulating T cells from head and neck squamous cell carcinoma (HNSCC) patients. Herein, we conducted experiments that elucidate the mechanisms of KCa3.1 dysfunction and impaired chemotaxis in HNSCC CD8+ T cells. The Ca2+ sensor calmodulin (CaM) controls multiple cellular functions including KCa3.1 activation. Our data showed that CaM expression is lower in HNSCC than healthy donor (HD) T cells. This reduction was due to an intrinsic decrease in the genes encoding CaM combined to the failure of HNSCC T cells to upregulate CaM upon activation. Furthermore, the reduction in CaM was confined to the plasma membrane and resulted in decreased CaM-KCa3.1 association and KCa3.1 activity (which was rescued by the delivery of CaM). IFN$\gamma$ production, also Ca2+- and CaM-dependent, was instead not reduced in HNSCC T cells, which maintained intact cytoplasmic CaM and Ca2+ fluxing ability. Knockdown of CaM in HD T cells decreased KCa3.1 activity, but not IFN$\gamma$ production, and reduced their chemotaxis in the presence of adenosine, thus recapitulating HNSCC T cell dysfunction. Activation of KCa3.1 with 1-EBIO restored the ability of CaM knockdown HD T cells to chemotax in the presence of adenosine. Additionally, 1-EBIO enhanced INF$\gamma$ production. Our data showed a localized downregulation of membrane-proximal CaM that suppressed KCa3.1 activity in HNSCC circulating T cells and limited their ability to infiltrate adenosine-rich tumor-like microenvironments. Furthermore, they indicate that KCa3.1 activators could be used as positive CD8+ T cell modulators in cancers.
Science immunology 2019 feb
Multifactorial heterogeneity of virus-specific T cells and association with the progression of human chronic hepatitis B infection.
Abstract
Abstract
Associations between chronic antigen stimulation, T cell dysfunction, and the expression of various inhibitory receptors are well characterized in several mouse and human systems. During chronic hepatitis B virus (HBV) infection (CHB), T cell responses are blunted with low frequencies of virus-specific T cells observed, making these parameters difficult to study. Here, using mass cytometry and a highly multiplexed combinatorial peptide-major histocompatibility complex (pMHC) tetramer strategy that allows for the detection of rare antigen-specific T cells, we simultaneously probed 484 unique HLA-A*1101-restricted epitopes spanning the entire HBV genome on T cells from patients at various stages of CHB. Numerous HBV-specific T cell populations were detected, validated, and profiled. T cells specific for two epitopes (HBVpol387 and HBVcore169) displayed differing and complex heterogeneities that were associated with the disease progression, and the expression of inhibitory receptors on these cells was not linearly related with their extent of T cell dysfunction. For HBVcore169-specific CD8+ T cells, we found cellular markers associated with long-term memory, polyfunctionality, and the presence of several previously unidentified public TCR clones that correlated with viral control. Using high-dimensional trajectory analysis of these cellular phenotypes, a pseudo-time metric was constructed that fit with the status of viral infection in corresponding patients. This was validated in a longitudinal cohort of patients undergoing antiviral therapy. Our study uncovers complex relationships of inhibitory receptors between the profiles of antigen-specific T cells and the status of CHB with implications for new strategies of therapeutic intervention.
Cancer research 2017 NOV
CD155T/TIGIT Signaling Regulates CD8+ T-cell Metabolism and Promotes Tumor Progression in Human Gastric Cancer.
Abstract
Abstract
The T-cell surface molecule TIGIT is an immune checkpoint molecule that inhibits T-cell responses, but its roles in cancer are little understood. In this study, we evaluated the role TIGIT checkpoint plays in the development and progression of gastric cancer. We show that the percentage of CD8 T cells that are TIGIT+ was increased in gastric cancer patients compared with healthy individuals. These cells showed functional exhaustion with impaired activation, proliferation, cytokine production, and metabolism, all of which were rescued by glucose. In addition, gastric cancer tissue and cell lines expressed CD155, which bound TIGIT receptors and inactivated CD8 T cells. In a T cell-gastric cancer cell coculture system, gastric cancer cells deprived CD8 T cells of glucose and impaired CD8 T-cell effector functions; these effects were neutralized by the additional glucose or by TIGIT blockade. In gastric cancer tumor cells, CD155 silencing increased T-cell metabolism and IFNγ production, whereas CD155 overexpression inhibited T-cell metabolism and IFNγ production; this inhibition was neutralized by TIGIT blockade. Targeting CD155/TIGIT enhanced CD8 T-cell reaction and improved survival in tumor-bearing mice. Combined targeting of TIGIT and PD-1 further enhanced CD8 T-cell activation and improved survival in tumor-bearing mice. Our results suggest that gastric cancer cells inhibit CD8 T-cell metabolism through CD155/TIGIT signaling, which inhibits CD8 T-cell effector functions, resulting in hyporesponsive antitumor immunity. These findings support the candidacy of CD155/TIGIT as a potential therapeutic target in gastric cancer. Cancer Res; 77(22); 6375-88. textcopyright2017 AACR.
Journal of immunology (Baltimore, Md. : 1950) 2016 MAR
Enhanced Cytotoxic CD8 T Cell Priming Using Dendritic Cell-Expressing Human Papillomavirus-16 E6/E7-p16INK4 Fusion Protein with Sequenced Anti-Programmed Death-1.
Abstract
Abstract
The incidence of human papillomavirus (HPV)-related head and neck squamous cell carcinoma has increased in recent decades, though HPV prevention vaccines may reduce this rise in the future. HPV-related cancers express the viral oncoproteins E6 and E7. The latter inactivates the tumor suppressor protein retinoblastoma (Rb), which leads to the overexpression of p16(INK4) protein, providing unique Ags for therapeutic HPV-specific cancer vaccination. We developed potential adenoviral vaccines that express a fusion protein of HPV-16 E6 and E7 (Ad.E6E7) alone or fused with p16 (Ad.E6E7p16) and also encoding an anti-programmed death (PD)-1 Ab. Human monocyte-derived dendritic cells (DC) transduced with Ad.E6E7 or Ad.E6E7p16 with or without Ad.αPD1 were used to activate autologous CD8 CTL in vitro. CTL responses were tested against naturally HPV-infected head and neck squamous cell carcinoma cells using IFN-γ ELISPOT and [(51)Cr]release assay. Surprisingly, stimulation and antitumor activity of CTL were increased after incubation with Ad.E6E7p16-transduced DC (DC.E6E7p16) compared with Ad.E6E7 (DC.E6E7), a result that may be due to an effect of p16 on cyclin-dependent kinase 4 levels and IL-12 secretion by DC. Moreover, the beneficial effect was most prominent when anti-PD-1 was introduced during the second round of stimulation (after initial priming). These data suggest that careful sequencing of Ad.E6E7.p16 with Ad.αPD1 could improve antitumor immunity against HPV-related tumors and that p16 may enhance the immunogenicity of DC, through cyclin-dependent pathways, Th1 cytokine secretion, and by adding a nonviral Ag highly overexpressed in HPV-induced cancers.
PloS one 2015
Application of the pMHC Array to Characterise Tumour Antigen Specific T Cell Populations in Leukaemia Patients at Disease Diagnosis.
Abstract
Abstract
Immunotherapy treatments for cancer are becoming increasingly successful, however to further improve our understanding of the T-cell recognition involved in effective responses and to encourage moves towards the development of personalised treatments for leukaemia immunotherapy, precise antigenic targets in individual patients have been identified. Cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen specific T-cell populations naturally circulating in patients and normal donors. We have developed the pMHC array to detect CD8+ T-cell populations in leukaemia patients that recognise epitopes within viral antigens (cytomegalovirus (CMV) and influenza (Flu)) and leukaemia antigens (including Per Arnt Sim domain 1 (PASD1), MelanA, Wilms' Tumour (WT1) and tyrosinase). We show that the pMHC array is at least as sensitive as flow cytometry and has the potential to rapidly identify more than 40 specific T-cell populations in a small sample of T-cells (0.8-1.4 x 10(6)). Fourteen of the twenty-six acute myeloid leukaemia (AML) patients analysed had T cells that recognised tumour antigen epitopes, and eight of these recognised PASD1 epitopes. Other tumour epitopes recognised were MelanA (n = 3), tyrosinase (n = 3) and WT1(126-134) (n = 1). One of the seven acute lymphocytic leukaemia (ALL) patients analysed had T cells that recognised the MUC1(950-958) epitope. In the future the pMHC array may be used provide point of care T-cell analyses, predict patient response to conventional therapy and direct personalised immunotherapy for patients.
Journal of immunology (Baltimore, Md. : 1950) 2010 MAR
Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes.
Abstract
Abstract
Increased proportions of CD8 T lymphocytes lacking expression of the CD28 costimulatory receptor have been documented during both aging and chronic infection with HIV-1, and their abundance correlates with numerous deleterious clinical outcomes. CD28-negative cells also arise in cell cultures of CD8(+)CD28(+) following multiple rounds of Ag-driven proliferation, reaching the end stage of replicative senescence. The present study investigates the role of a second T cell costimulatory receptor component, adenosine deaminase (ADA), on the process of replicative senescence. We had previously reported that CD28 signaling is required for optimal telomerase upregulation. In this study, we show that the CD8(+)CD28(+) T lymphocytes that are ADA(+) have significantly greater telomerase activity than those that do not express ADA and that ADA is progressively lost as cultures progress to senescence. Because ADA converts adenosine to inosine, cells lacking this enzyme might be subject to prolonged exposure to adenosine, which has immunosuppressive effects. Indeed, we show that chronic exposure of CD8 T lymphocytes to exogenous adenosine accelerates the process of replicative senescence, causing a reduction in overall proliferative potential, reduced telomerase activity, and blunted IL-2 gene transcription. The loss of CD28 expression was accelerated, in part due to adenosine-induced increases in constitutive caspase-3, known to act on the CD28 promoter. These findings provide the first evidence for a role of ADA in modulating the process of replicative senescence and suggest that strategies to enhance this enzyme may lead to novel therapeutic approaches for pathologies associated with increases in senescent CD8 T lymphocytes.