EasySep™ Human CD8 Positive Selection Kit II
Immunomagnetic positive selection kit
概要
The EasySep™ Human CD8 Positive Selection Kit II is designed to isolate CD8+ cells from fresh or previously frozen peripheral blood mononuclear cells or washed leukapheresis samples by immunomagnetic positive selection. Desired cells are targeted with antibody complexes recognizing CD8 and magnetic particles. The cocktail also contains an antibody to human Fc receptor to minimize nonspecific binding. Labeled cells are separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off. The CD8 antigen is expressed on cytotoxic T cells and weakly on a subset of NK cells.
This product replaces the EasySep™ Human CD8 Positive Selection Kit (Catalog #18053) for even faster cell isolations.
This product replaces the EasySep™ Human CD8 Positive Selection Kit (Catalog #18053) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 99% purity
• No columns required
• Up to 99% purity
• No columns required
Components
- EasySep™ Human CD8 Positive Selection Kit II (Catalog #17853)
- EasySep™ Human CD8 Positive Selection Cocktail II, 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Human CD8 Positive Selection Kit II (Catalog #17853RF)
- EasySep™ Human CD8 Positive Selection Cocktail II, 1 mL
- EasySep™ Dextran RapidSpheres™ 50100, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyPlate™ EasySep™ Magnet (Catalog #18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD8+
Species
Human
Sample Source
PBMC
Selection Method
Positive
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD8 Positive Selection Kit II | 17853 | All | English |
| Product Information Sheet | RoboSep™ Human CD8 Positive Selection Kit II | 17853RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD8 Positive Selection Kit II | 17853 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD8 Positive Selection Kit II | 17853 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD8 Positive Selection Kit II | 17853RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD8 Positive Selection Kit II | 17853RF | All | English |
数据及文献
Data
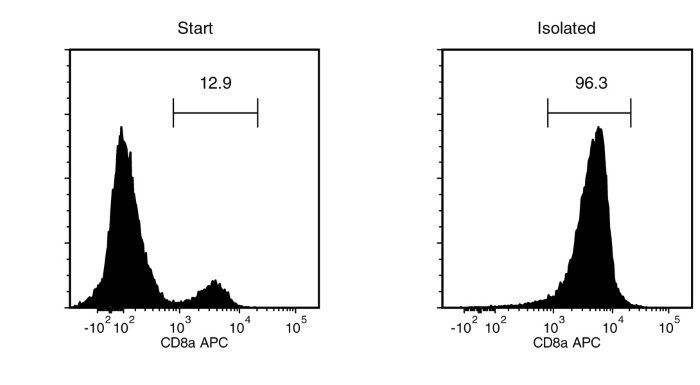
Figure 1. Typical EasySep™ Human CD8 Positive Selection Profile
Starting with a single cell suspension of human PBMCs, the CD8+ cell content of the isolated fraction is typically 96.5 ± 2.4% (mean ± SD using "The Big Easy" EasySep™ Magnet).
Publications (8)
Scientific reports 2020 mar
Assessing the impact of AGS-004, a dendritic cell-based immunotherapy, and vorinostat on persistent HIV-1 Infection.
Abstract
Abstract
Approaches to deplete persistent HIV infection are needed. We investigated the combined impact of the latency reversing agent vorinostat (VOR) and AGS-004, an autologous dendritic cell immunotherapeutic, on the HIV reservoir. HIV+, stably treated participants in whom resting CD4+ T cell-associated HIV RNA (rca-RNA) increased after VOR exposure ex vivo and in vivo received 4 doses of AGS-004 every 3 weeks, followed by VOR every 72 hours for 30 days, and then the cycle repeated. Change in VOR-responsive host gene expression, HIV-specific T cell responses, low-level HIV viremia, rca-RNA, and the frequency of resting CD4+ T-cell infection (RCI) was measured at baseline and after each cycle. No serious treatment-related adverse events were observed among five participants. As predicted, VOR-responsive host genes responded uniformly to VOR dosing. Following cycles of AGS-004 and VOR, rca-RNA decreased significantly in only two participants, with a significant decrease in SCA observed in one of these participants. However, unlike other cohorts dosed with AGS-004, no uniform increase in HIV-specific immune responses following vaccination was observed. Finally, no reproducible decline of RCI, defined as a decrease of {\textgreater}50{\%}, was observed. AGS-004 and VOR were safe and well-tolerated, but no substantial impact on RCI was measured. In contrast to previous clinical data, AGS-004 did not induce HIV-specific immune responses greater than those measured at baseline. More efficacious antiviral immune interventions, perhaps paired with more effective latency reversal, must be developed to clear persistent HIV infection.
Science advances 2019 jan
Multiplexed enrichment and genomic profiling of peripheral blood cells reveal subset-specific immune signatures.
Abstract
Abstract
Specialized immune cell subsets are involved in autoimmune disease, cancer immunity, and infectious disease through a diverse range of functions mediated by overlapping pathways and signals. However, subset-specific responses may not be detectable in analyses of whole blood samples, and no efficient approach for profiling cell subsets at high throughput from small samples is available. We present a low-input microfluidic system for sorting immune cells into subsets and profiling their gene expression. We validate the system's technical performance against standard subset isolation and library construction protocols and demonstrate the importance of subset-specific profiling through in vitro stimulation experiments. We show the ability of this integrated platform to identify subset-specific disease signatures by profiling four immune cell subsets in blood from patients with systemic lupus erythematosus (SLE) and matched control subjects. The platform has the potential to make multiplexed subset-specific analysis routine in many research laboratories and clinical settings.
Nature communications 2019 feb
PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals.
Abstract
Abstract
HIV persists in latently infected CD4+ T cells during antiretroviral therapy (ART). Immune checkpoint molecules, including PD-1, are preferentially expressed at the surface of persistently infected cells. However, whether PD-1 plays a functional role in HIV latency and reservoir persistence remains unknown. Using CD4+ T cells from HIV-infected individuals, we show that the engagement of PD-1 inhibits viral production at the transcriptional level and abrogates T-cell receptor (TCR)-induced HIV reactivation in latently infected cells. Conversely, PD-1 blockade with the monoclonal antibody pembrolizumab enhances HIV production in combination with the latency reversing agent bryostatin without increasing T cell activation. Our results suggest that the administration of immune checkpoint blockers to HIV-infected individuals on ART may facilitate latency disruption.
The Journal of clinical investigation 2019 dec
Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations.
Abstract
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease with no known cause or mechanism. There is an increasing appreciation for the role of immune and metabolic dysfunction in the disease. ME/CFS has historically presented in outbreaks, often has a flu-like onset, and results in inflammatory symptoms. Patients suffer from severe fatigue and post-exertional malaise. There is little known about the metabolism of specific immune cells in ME/CFS patients. To investigate immune metabolism in ME/CFS, we isolated CD4+ and CD8+ T cells from 53 ME/CFS patients and 45 healthy controls. We analyzed glycolysis and mitochondrial respiration in resting and activated T cells, along with markers related to cellular metabolism, and plasma cytokines. We found that ME/CFS CD8+ T cells have reduced mitochondrial membrane potential compared to healthy controls. Both CD4+ and CD8+ T cells from ME/CFS patients had reduced glycolysis at rest, while CD8+ T cells also had reduced glycolysis following activation. ME/CFS patients had significant correlations between measures of T cell metabolism and plasma cytokine abundance that differed from healthy control subjects. Our data indicate that patients have impaired T cell metabolism consistent with ongoing immune alterations in ME/CFS that may illuminate the mechanism behind this disease.
Cells 2019 apr
Chronic Hepatitis C Virus Infection Impairs M1 Macrophage Differentiation and Contributes to CD8+ T-Cell Dysfunction.
Abstract
Abstract
Chronic hepatitis C virus (HCV) infection causes generalized CD8+ T cell impairment, not limited to HCV-specific CD8+ T-cells. Liver-infiltrating monocyte-derived macrophages (MDMs) contribute to the local micro-environment and can interact with and influence cells routinely trafficking through the liver, including CD8+ T-cells. MDMs can be polarized into M1 (classically activated) and M2a, M2b, and M2c (alternatively activated) phenotypes that perform pro- and anti-inflammatory functions, respectively. The impact of chronic HCV infection on MDM subset functions is not known. Our results show that M1 cells generated from chronic HCV patients acquire M2 characteristics, such as increased CD86 expression and IL-10 secretion, compared to uninfected controls. In contrast, M2 subsets from HCV-infected individuals acquired M1-like features by secreting more IL-12 and IFN-gamma. The severity of liver disease was also associated with altered macrophage subset differentiation. In co-cultures with autologous CD8+ T-cells from controls, M1 macrophages alone significantly increased CD8+ T cell IFN-gamma expression in a cytokine-independent and cell-contact-dependent manner. However, M1 macrophages from HCV-infected individuals significantly decreased IFN-gamma expression in CD8+ T-cells. Therefore, altered M1 macrophage differentiation in chronic HCV infection may contribute to observed CD8+ T-cell dysfunction. Understanding the immunological perturbations in chronic HCV infection will lead to the identification of therapeutic targets to restore immune function in HCV+ individuals, and aid in the mitigation of associated negative clinical outcomes.
Nature communications 2018 OCT
Human breast tumor-infiltrating CD8+ T cells retain polyfunctionality despite PD-1 expression.
Abstract
Abstract
Functional CD8+ T cells in human tumors play a clear role in clinical prognosis and response to immunotherapeutic interventions. PD-1 expression in T cells involved in chronic infections and tumors such as melanoma often correlates with a state of T-cell exhaustion. Here we interrogate CD8+ tumor-infiltrating lymphocytes (TILs) from human breast and melanoma tumors to explore their functional state. Despite expression of exhaustion hallmarks, such as PD-1 expression, human breast tumor CD8+ TILs retain robust capacity for production of effector cytokines and degranulation capacity. In contrast, melanoma CD8+ TILs display dramatic reduction of cytokine production and degranulation capacity. We show that CD8+ TILs from human breast tumors can potently kill cancer cells via bi-specific antibodies. Our data demonstrate that CD8+ TILs in human breast tumors retain polyfunctionality, despite PD-1 expression, and suggest that they may be harnessed for effective immunotherapies.