EasySep™ Human CD4+ T Cell Enrichment Kit
Immunomagnetic negative selection kit
概要
The EasySep™ Human CD4+ T Cell Enrichment Kit is designed to isolate CD4+ T cells from fresh or previously frozen peripheral mononuclear cells by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-CD4+ T cells and dextran-coated magnetic particles. Labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
For even faster cell isolations, we recommend the new EasySep™ Human CD4+ T Cell Isolation Kit (17952) which isolates cells in just 8 minutes.
For even faster cell isolations, we recommend the new EasySep™ Human CD4+ T Cell Isolation Kit (17952) which isolates cells in just 8 minutes.
Advantages
• Fast, easy-to-use and column-free
• Up to 97% purity
• Untouched, viable cells
• Up to 97% purity
• Untouched, viable cells
Components
- EasySep™ Human CD4+ T Cell Enrichment Kit (Catalog #19052)
- EasySep™ Human CD4+ T Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- RoboSep™ Human CD4+ T Cell Enrichment Kit with Filter Tips (Catalog #19052RF)
- EasySep™ Human CD4+ T Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD4+
Species
Human
Sample Source
Leukapheresis, PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human CD4+ T Cell Enrichment Kit | 19052 | All | English |
| Product Information Sheet | RoboSep™ Human CD4+ T Cell Enrichment Kit with Filter Tips | 19052RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD4+ T Cell Enrichment Kit | 19052 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD4+ T Cell Enrichment Kit | 19052 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD4+ T Cell Enrichment Kit with Filter Tips | 19052RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD4+ T Cell Enrichment Kit with Filter Tips | 19052RF | All | English |
数据及文献
Data
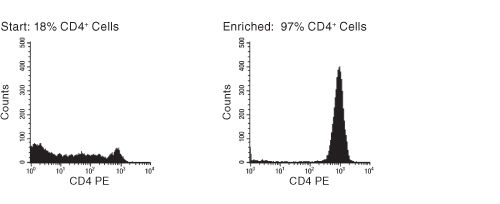
Figure 1. FACS Histogram Results Using EasySep™ Human CD4+ T Cell Enrichment Kit
Starting with frozen mononuclear cells, the CD4+ T cell content of the enriched fraction typically ranges from 92% - 97%.
Publications (45)
Nature immunology 2020 nov
Functional CRISPR dissection of gene networks controlling human regulatory T cell identity.
Abstract
Abstract
Human regulatory T (Treg) cells are essential for immune homeostasis. The transcription factor FOXP3 maintains Treg cell identity, yet the complete set of key transcription factors that control Treg cell gene expression remains unknown. Here, we used pooled and arrayed Cas9 ribonucleoprotein screens to identify transcription factors that regulate critical proteins in primary human Treg cells under basal and proinflammatory conditions. We then generated 54,424 single-cell transcriptomes from Treg cells subjected to genetic perturbations and cytokine stimulation, which revealed distinct gene networks individually regulated by FOXP3 and PRDM1, in addition to a network coregulated by FOXO1 and IRF4. We also discovered that HIVEP2, to our knowledge not previously implicated in Treg cell function, coregulates another gene network with SATB1 and is important for Treg cell-mediated immunosuppression. By integrating CRISPR screens and single-cell RNA-sequencing profiling, we have uncovered transcriptional regulators and downstream gene networks in human Treg cells that could be targeted for immunotherapies.
European journal of medicinal chemistry 2020 jan
Tuning isoform selectivity and bortezomib sensitivity with a new class of alkenyl indene PDI inhibitor.
Abstract
Abstract
Protein disulfide isomerase (PDI, PDIA1) is an emerging therapeutic target in oncology. PDI inhibitors have demonstrated a unique propensity to selectively induce apoptosis in cancer cells and overcome resistance to existing therapies, although drug candidates have not yet progressed to the stage of clinical development. We recently reported the discovery of lead indene compound E64FC26 as a potent pan-PDI inhibitor that enhances the cytotoxic effects of proteasome inhibitors in panels of Multiple Myeloma (MM) cells and MM mouse models. An extensive medicinal chemistry program has led to the generation of a diverse library of indene-containing molecules with varying degrees of proteasome inhibitor potentiating activity. These compounds were generated by a novel nucleophilic aromatic ring cyclization and dehydration reaction from the precursor ketones. The results provide detailed structure activity relationships (SAR) around this indene pharmacophore and show a high degree of correlation between potency of PDI inhibition and bortezomib (Btz) potentiation in MM cells. Inhibition of PDI leads to ER and oxidative stress characterized by the accumulation of misfolded poly-ubiquitinated proteins and the induction of UPR biomarkers ATF4, CHOP, and Nrf2. This work characterizes the synthesis and SAR of a new chemical class and further validates PDI as a therapeutic target in MM as a single agent and in combination with proteasome inhibitors.
Scientific reports 2019 mar
Hexamethylene bisacetamide impairs NK cell-mediated clearance of acute T lymphoblastic leukemia cells and HIV-1-infected T cells that exit viral latency.
Abstract
Abstract
The hexamethylene bisacetamide (HMBA) anticancer drug was dismissed due to limited efficacy in leukemic patients but it may re-enter into the clinics in HIV-1 eradication strategies because of its recently disclosed capacity to reactivate latent virus. Here, we investigated the impact of HMBA on the cytotoxicity of natural killer (NK) cells against acute T lymphoblastic leukemia (T-ALL) cells or HIV-1-infected T cells that exit from latency. We show that in T-ALL cells HMBA upmodulated MICB and ULBP2 ligands for the NKG2D activating receptor. In a primary CD4+ T cell-based latency model, HMBA did not reactivate HIV-1, yet enhanced ULBP2 expression on cells harboring virus reactivated by prostratin (PRO). However, HMBA reduced the expression of NKG2D and its DAP10 adaptor in NK cells, hence impairing NKG2D-mediated cytotoxicity and DAP10-dependent response to IL-15 stimulation. Alongside, HMBA dampened killing of T-ALL targets by IL-15-activated NK cells and impaired NK cell-mediated clearance of PRO-reactivated HIV-1+ cells. Overall, our results demonstrate a dominant detrimental effect of HMBA on the NKG2D pathway that crucially controls NK cell-mediated killing of tumors and virus-infected cells, providing one possible explanation for poor clinical outcome in HMBA-treated cancer patients and raising concerns for future therapeutic application of this drug.
Scientific reports 2019 mar
Activation of Peripheral Blood CD4+ T-Cells in IBS is not Associated with Gastrointestinal or Psychological Symptoms.
Abstract
Abstract
Immune activation may underlie the pathogenesis of irritable bowel syndrome (IBS), but the evidence is conflicting. We examined whether peripheral CD4+ T-cells from IBS patients demonstrated immune activation and changes in cytokine production. To gain mechanistic insight, we examined whether immune activation correlated with psychological stress and changing symptoms over time. IBS patients (n = 29) and healthy volunteers (HV; n = 29) completed symptom and psychological questionnaires. IBS patients had a significant increase in CD4+ T-cells expressing the gut homing marker integrin beta7 (p = 0.023) and lymphoid marker CD62L (p = 0.026) compared to HV. Furthermore, phytohaemagglutinin stimulated CD4+ T-cells from IBS-D patients demonstrated increased TNFalpha secretion when compared to HV (p = 0.044). Increased psychological scores in IBS did not correlate with TNFalpha production, while stress hormones inhibited cytokine secretion from CD4+ T-cells of HV in vitro. IBS symptoms, but not markers of immune activation, decreased over time. CD4+ T-cells from IBS-D patients exhibit immune activation, but this did not appear to correlate with psychological stress measurements or changing symptoms over time. This could suggest that immune activation is a surrogate of an initial trigger and/or ongoing parallel peripheral mechanisms.
Journal of immunology (Baltimore, Md. : 1950) 2019 jun
TRAILshort Protects against CD4 T Cell Death during Acute HIV Infection.
Abstract
Abstract
CD4 T cells from HIV-1 infected patients die at excessive rates compared to those from uninfected patients, causing immunodeficiency. We previously identified a dominant negative ligand that antagonizes the TRAIL-dependent pathway of cell death, which we called TRAILshort. Because the TRAIL pathway has been implicated in CD4 T cell death occurring during HIV-1 infection, we used short hairpin RNA knockdown, CRISPR deletion, or Abs specific for TRAILshort to determine the effect of inhibiting TRAILshort on the outcome of experimental acute HIV infection in vitro. Strikingly, all three approaches to TRAILshort deletion/inhibition enhanced HIV-induced death of both infected and uninfected human CD4 T cells. Thus, TRAILshort impacts T cell dynamics during HIV infection, and inhibiting TRAILshort causes more HIV-infected and uninfected bystander cells to die. TRAILshort is, therefore, a host-derived, host-adaptive mechanism to limit the effects of TRAIL-induced cell death. Further studies on the effects of TRAILshort in other disease states are warranted.
Journal of immunology (Baltimore, Md. : 1950) 2019 jul
Metformin Inhibits the Type 1 IFN Response in Human CD4+ T Cells.
Abstract
Abstract
In systemic lupus erythematosus, defective clearance of apoptotic debris and activation of innate cells result in a chronically activated type 1 IFN response, which can be measured in PBMCs of most patients. Metformin, a widely used prescription drug for Type 2 diabetes, has a therapeutic effect in several mouse models of lupus through mechanisms involving inhibition of oxidative phosphorylation and a decrease in CD4+ T cell activation. In this study, we report that in CD4+ T cells from human healthy controls and human systemic lupus erythematosus patients, metformin inhibits the transcription of IFN-stimulated genes (ISGs) after IFN-alpha treatment. Accordingly, metformin inhibited the phosphorylation of pSTAT1 (Y701) and its binding to IFN-stimulated response elements that control ISG expression. These effects were independent of AMPK activation or mTORC1 inhibition but were replicated using inhibitors of the electron transport chain respiratory complexes I, III, and IV. This indicates that mitochondrial respiration is required for ISG expression in CD4+ T cells and provides a novel mechanism by which metformin may exert a therapeutic effect in autoimmune diseases.