EasySep™ Mouse T Cell Isolation Kit
15-Minute cell isolation kit using immunomagnetic negative selection
概要
The EasySep™ Mouse T Cell Isolation Kit is designed to isolate T cells from single-cell suspensions of splenocytes or other tissues by negative selection. Unwanted cells are targeted for removal with biotinylated antibodies directed against non-T cells and streptavidin-coated magnetic particles. Labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
This product replaces the EasySep™ Mouse T Cell Enrichment Kit (Catalog #19751) for even faster cell isolations.
This product replaces the EasySep™ Mouse T Cell Enrichment Kit (Catalog #19751) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 99% purity
• No columns required
• Untouched, viable cells
• Up to 99% purity
• No columns required
• Untouched, viable cells
Components
- EasySep™ Mouse T Cell Isolation Kit (Catalog #19851)
- EasySep™ Mouse T Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 1 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Mouse T Cell Isolation Kit (Catalog #19851RF)
- EasySep™ Mouse T Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 1.0 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells
Species
Mouse
Sample Source
Other, Spleen
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse T Cell Isolation Kit | 19851 | All | English |
| Product Information Sheet | RoboSep™ Mouse T Cell Isolation Kit | 19851RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse T Cell Isolation Kit | 19851 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse T Cell Isolation Kit | 19851 | All | English |
| Safety Data Sheet 3 | EasySep™ Mouse T Cell Isolation Kit | 19851 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse T Cell Isolation Kit | 19851RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse T Cell Isolation Kit | 19851RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Mouse T Cell Isolation Kit | 19851RF | All | English |
数据及文献
Data
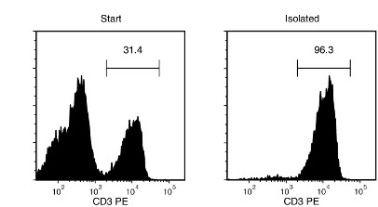
Figure 1. Typical EasySep™ Mouse T Cell Isolation Profile
Starting with mouse splenocytes, the T cell content of the isolated fraction typically ranges from 91.7 - 98.6%.
Publications (18)
Scientific reports 2020 jul
Bioluminescence for in vivo detection of cell-type-specific inflammation in a mouse model of uveitis.
Abstract
Abstract
This study reports the use of cell-type-specific in vivo bioluminescence to measure intraocular immune cell population dynamics during the course of inflammation in a mouse model of uveitis. Transgenic lines expressing luciferase in inflammatory cell subsets (myeloid cells, T cells, and B cells) were generated and ocular bioluminescence was measured serially for 35 days following uveitis induction. Ocular leukocyte populations were identified using flow cytometry and compared to the ocular bioluminescence profile. Acute inflammation is neutrophilic (75{\%} of ocular CD45 + cells) which is reflected by a significant increase in ocular bioluminescence in one myeloid reporter line on day 2. By day 7, the ocular T cell population increases to 50{\%} of CD45 + cells, leading to a significant increase in ocular bioluminescence in the T cell reporter line. While initially negligible ({\textless} 1{\%} of CD45 + cells), the ocular B cell population increases to {\textgreater} 4{\%} by day 35. This change is reflected by a significant increase in the ocular bioluminescence of the B cell reporter line starting on day 28. Our data demonstrates that cell-type-specific in vivo bioluminescence accurately detects changes in multiple intraocular immune cell populations over time in experimental uveitis. This assay could also be useful in other inflammatory disease models.
Nature 2020
VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours.
Abstract
Abstract
Immune surveillance against pathogens and tumours in the central nervous system is thought to be limited owing to the lack of lymphatic drainage. However, the characterization of the meningeal lymphatic network has shed light on previously unappreciated ways that an immune response can be elicited to antigens that are expressed in the brain1-3. Despite progress in our understanding of the development and structure of the meningeal lymphatic system, the contribution of this network in evoking a protective antigen-specific immune response in the brain remains unclear. Here, using a mouse model of glioblastoma, we show that the meningeal lymphatic vasculature can be manipulated to mount better immune responses against brain tumours. The immunity that is mediated by CD8 T cells to the glioblastoma antigen is very limited when the tumour is confined to the central nervous system, resulting in uncontrolled tumour growth. However, ectopic expression of vascular endothelial growth factor C (VEGF-C) promotes enhanced priming of CD8 T cells in the draining deep cervical lymph nodes, migration of CD8 T cells into the tumour, rapid clearance of the glioblastoma and a long-lasting antitumour memory response. Furthermore, transfection of an mRNA construct that expresses VEGF-C works synergistically with checkpoint blockade therapy to eradicate existing glioblastoma. These results reveal the capacity of VEGF-C to promote immune surveillance of tumours, and suggest a new therapeutic approach to treat brain tumours.
Cancer research 2020
ICOS Is an Indicator of T-cell-Mediated Response to Cancer Immunotherapy.
Abstract
Abstract
Immunotherapy is innovating clinical cancer management. Nevertheless, only a small fraction of patient's benefit from current immunotherapies. To improve clinical management of cancer immunotherapy, it is critical to develop strategies for response monitoring and prediction. In this study, we describe inducible T-cell costimulator (ICOS) as a conserved mediator of immune response across multiple therapy strategies. ICOS expression was evaluated by flow cytometry, 89Zr-DFO-ICOS mAb PET/CT imaging was performed on Lewis lung cancer models treated with different immunotherapy strategies, and the change in tumor volume was used as a read-out for therapeutic response. ImmunoPET imaging of ICOS enabled sensitive and specific detection of activated T cells and early benchmarking of immune response. A STING (stimulator of interferon genes) agonist was identified as a promising therapeutic approach in this manner. The STING agonist generated significantly stronger immune responses as measured by ICOS ImmunoPET and delayed tumor growth compared with programmed death-1 checkpoint blockade. More importantly, ICOS ImmunoPET enabled early and robust prediction of therapeutic response across multiple treatment regimens. These data show that ICOS is an indicator of T-cell-mediated immune response and suggests ICOS ImmunoPET as a promising strategy for monitoring, comparing, and predicting immunotherapy success in cancer. SIGNIFICANCE: ICOS ImmunoPET is a promising strategy to noninvasively predict and monitor immunotherapy response.See related commentary by Choyke, p. 2975.
Scientific reports 2019 nov
ATP amplifies NADPH-dependent and -independent neutrophil extracellular trap formation.
Abstract
Abstract
Neutrophils are the first immune cells to kill invading microbes at sites of infection using a variety of processes, including the release of proteases, phagocytosis and the production of neutrophil extracellular traps (NETs). NET formation, or NETosis, is a specific and highly efficient process, which is induced by a variety of stimuli leading to expulsion of DNA, proteases and antimicrobial peptides to the extracellular space. However, uncontrolled NETosis may lead to adverse effects and exert tissue damage in pathological conditions. Here, we show that the ATP channel pannexin1 (Panx1) is functionally expressed by bone marrow-derived neutrophils (BMDNs) of wild-type (WT) mice and that ATP contributes to NETosis induced in vitro by the calcium ionophore A23187 or phorbol 12-myristate 13-acetate (PMA). Interestingly, neutrophils isolated from Panx1-/- mice showed reduced and/or delayed induction of NETosis. Brilliant blue FCF dye (BB-FCF), a Panx1 channel inhibitor, decreased NETosis in wild-type neutrophils to the extent observed in Panx1-/- neutrophils. Thus, we demonstrate that ATP and Panx1 channels contribute to NETosis and may represent a therapeutic target.
Molecular therapy : the journal of the American Society of Gene Therapy 2019 nov
Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy.
Abstract
Abstract
Exosomes are nanosized membranous vesicles secreted by a variety of cells. Due to their unique and pharmacologically important properties, cell-derived exosome nanoparticles have drawn significant interest for drug development. By genetically modifying exosomes with two distinct types of surface-displayed monoclonal antibodies, we have developed an exosome platform termed synthetic multivalent antibodies retargeted exosome (SMART-Exo) for controlling cellular immunity. Here, we apply this approach to human epidermal growth factor receptor 2 (HER2)-expressing breast cancer by engineering exosomes through genetic display of both anti-human CD3 and anti-human HER2 antibodies, resulting in SMART-Exos dually targeting T cell CD3 and breast cancer-associated HER2 receptors. By redirecting and activating cytotoxic T cells toward attacking HER2-expressing breast cancer cells, the designed SMART-Exos exhibited highly potent and specific anti-tumor activity both in vitro and in vivo. This work demonstrates preclinical feasibility of utilizing endogenous exosomes for targeted breast cancer immunotherapy and the SMART-Exos as a broadly applicable platform technology for the development of next-generation immuno-nanomedicines.
NPJ vaccines 2019
Role of innate lymphoid cells and dendritic cells in intradermal immunization of the enterovirus antigen.
Abstract
Abstract
Enterovirus type 71 (EV71) and coxsackievirus A 16 (CA16) are the major pathogens of human hand, foot, and mouth disease (HFMD). In our previous study, intramuscular immunization with the inactivated EV71 vaccine elicited effective immunity, while immunization with the inactivated CA16 vaccine did not. In this report, we focused on innate immune responses elicited by inactivated EV71 and CA16 antigens administered intradermally or intramuscularly. The distributions of the EV71 and CA16 antigens administered intradermally or intramuscularly were not obviously different, but the antigens were detected for a shorter period of time when administered intradermally. The expression levels of NF-kappaB pathway signaling molecules, which were identified as being capable of activating DCs, ILCs, and T cells, were higher in the intradermal group than in the intramuscular group. Antibodies for the EV71 and CA16 antigens colocalized with ILCs and DCs in skin and muscle tissues under fluorescence microscopy. Interestingly, ILC colocalization decreased over time, while DC colocalization increased over time. ELISpot analysis showed that coordination between DCs and ILCs contributed to successful adaptive immunity against vaccine antigens in the skin. EV71 and/or CA16 antigen immunization via the intradermal route was more capable of significantly increasing neutralizing antibody titers and activating specific T cell responses than immunization via the intramuscular route. Furthermore, neonatal mice born to mothers immunized with the EV71 and CA16 antigens were 100{\%} protected against wild-type EV71 or CA16 viral challenge. Together, our results provide new insights into the development of vaccines for HFMD.