EasySep™ Human B Cell Enrichment Kit II Without CD43 Depletion
Immunomagnetic negative selection kit
概要
The EasySep™ Human B Cell Enrichment Kit II without CD43 Depletion is designed to isolate B cells by negative selection from fresh or previously frozen PBMCs of individuals with B cell leukemia or lymphoma, or other disease states in which B cells may express CD43. The EasySep™ procedure involves labeling unwanted cells with antibody complexes and magnetic particles. The magnetically labeled cells are separated from the untouched desired cells by using an EasySep™ magnet and simply pouring or pipetting the desired cells into a new tube.
This kit replaces the EasySep™ Human B Cell Enrichment Kit without CD43 (Catalog #19154) for even faster cell isolations.
This kit replaces the EasySep™ Human B Cell Enrichment Kit without CD43 (Catalog #19154) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 99% purity
• Isolated cells are untouched
• Up to 99% purity
• Isolated cells are untouched
Components
- EasySep™ Human B Cell Enrichment Kit II without CD43 Depletion (Catalog #17963)
- EasySep™ Human B Cell Enrichment Cocktail II without CD43 Depletion, 1 mL
- EasySep™ Isolation Cocktail Enhancer, 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Human B Cell Enrichment Kit II without CD43 Depletion (Catalog #17963RF)
- EasySep™ Human B Cell Enrichment Cocktail II without CD43 Depletion, 1 mL
- EasySep™ Isolation Cocktail Enhancer, 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
B Cells
Species
Human
Sample Source
PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
数据及文献
Data
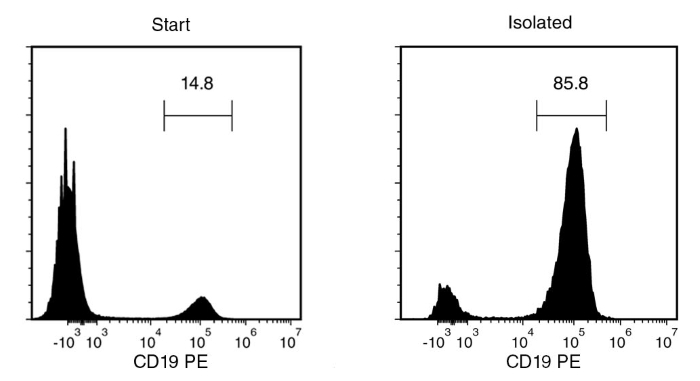
Starting with fresh PBMCs, the B cell content (CD19+) of the enriched fraction is typically 84.9 ± 13.9% (mean ± SD using the purple EasySep™ Magnet). In the above example, the purities of the start and final enriched fractions are 14.8% and 85.8%, respectively.
Publications (2)
The Journal of experimental medicine 2020 sep
Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with mantle cell lymphoma: A phase I/II trial.
Abstract
Abstract
Here, we report on the results of a phase I/II trial (NCT00490529) for patients with mantle cell lymphoma who, having achieved remission after immunochemotherapy, were vaccinated with irradiated, CpG-activated tumor cells. Subsequently, vaccine-primed lymphocytes were collected and reinfused after a standard autologous stem cell transplantation (ASCT). The primary endpoint was detection of minimal residual disease (MRD) within 1 yr after ASCT at the previously validated threshold of ≥1 malignant cell per 10,000 leukocyte equivalents. Of 45 evaluable patients, 40 (89{\%}) were found to be MRD negative, and the MRD-positive patients experienced early subsequent relapse. The vaccination induced antitumor CD8 T cell immune responses in 40{\%} of patients, and these were associated with favorable clinical outcomes. Patients with high tumor PD-L1 expression after in vitro exposure to CpG had inferior outcomes. Vaccination with CpG-stimulated autologous tumor cells followed by the adoptive transfer of vaccine-primed lymphocytes after ASCT is feasible and safe.
Scientific reports 2020
Time to first treatment and P53 dysfunction in chronic lymphocytic leukaemia: results of the O-CLL1 study in early stage patients.
Abstract
Abstract
Chronic lymphocytic leukaemia (CLL) is characterised by a heterogeneous clinical course. Such heterogeneity is associated with a number of markers, including TP53 gene inactivation. While TP53 gene alterations determine resistance to chemotherapy, it is not clear whether they can influence early disease progression. To clarify this issue, TP53 mutations and deletions of the corresponding locus [del(17p)] were evaluated in 469 cases from the O-CLL1 observational study that recruited a cohort of clinically and molecularly characterised Binet stage A patients. Twenty-four cases harboured somatic TP53 mutations [accompanied by del(17p) in 9 cases], 2 patients had del(17p) only, and 5 patients had TP53 germ-line variants. While del(17p) with or without TP53 mutations was capable of significantly predicting the time to first treatment, a reliable measure of disease progression, TP53 mutations were not. This was true for cases with high or low variant allele frequency. The lack of predictive ability was independent of the functional features of the mutant P53 protein in terms of transactivation and dominant negative potential. TP53 mutations alone were more frequent in patients with mutated IGHV genes, whereas del(17p) was associated with the presence of adverse prognostic factors, including CD38 positivity, unmutated-IGHV gene status, and NOTCH1 mutations.