EasySep™ Human B Cell Enrichment Kit
Immunomagnetic negative selection kit
概要
The EasySep™ Human B Cell Enrichment Kit is designed to isolate B cells from fresh or previously frozen peripheral blood mononuclear cells by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-B cells and dextran-coated magnetic particles. The labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
For even faster cell isolations, we recommend the new EasySep™ Human B Cell Isolation Kit (17954), which isolates cells in just 9 minutes.
For even faster cell isolations, we recommend the new EasySep™ Human B Cell Isolation Kit (17954), which isolates cells in just 9 minutes.
Advantages
• Fast, easy-to-use and column-free
• Up to 99% purity
• Untouched, viable cells
• Up to 99% purity
• Untouched, viable cells
Components
- EasySep™ Human B Cell Enrichment Kit (Catalog #19054)
- EasySep™ Human B Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- RoboSep™ Human B Cell Enrichment Kit with Filter Tips (Catalog #19054RF)
- EasySep™ Human B Cell Enrichment Cocktail, 1 mL
- EasySep™ D Magnetic Particles, 2 x 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
B Cells
Species
Human
Sample Source
Leukapheresis, PBMC
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human B Cell Enrichment Kit | 19054 | All | English |
| Product Information Sheet | RoboSep™ Human B Cell Enrichment Kit with Filter Tips | 19054RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human B Cell Enrichment Kit | 19054 | All | English |
| Safety Data Sheet 2 | EasySep™ Human B Cell Enrichment Kit | 19054 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human B Cell Enrichment Kit with Filter Tips | 19054RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human B Cell Enrichment Kit with Filter Tips | 19054RF | All | English |
数据及文献
Data
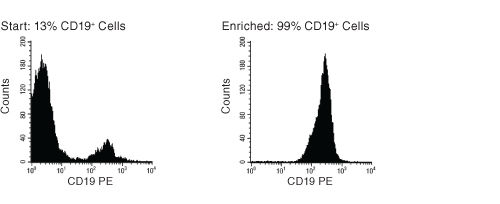
Figure 1. FACS Histogram Results With EasySep™ Human B Cell Enrichment Kit
Starting with frozen mononuclear cells, the CD19+ cell content of the enriched fraction typically ranges from 95% - 99%.
Publications (17)
Cell reports 2020 oct
Interferon-$\lambda$ Enhances the Differentiation of Naive B Cells into Plasmablasts via the mTORC1 Pathway.
Abstract
Abstract
Type III interferon (interferon lambda [IFN-$\lambda$]) is known to be a potential immune modulator, but the mechanisms behind its immune-modulatory functions and its impact on plasmablast differentiation in humans remain unknown. Human B cells and their subtypes directly respond to IFN-$\lambda$. Using B cell transcriptome profiling, we investigate the immune-modulatory role of IFN-$\lambda$ in B cells. We find that IFN-$\lambda$-induced gene expression in B cells is steady, prolonged, and importantly, cell type specific. Furthermore, IFN-$\lambda$ enhances the mTORC1 (mammalian/mechanistic target of rapamycin complex 1) pathway in B cells activated by the B cell receptor (BCR/anti-IgM). Engagement of mTORC1 by BCR and IFN-$\lambda$ induces cell-cycle progress in B cells. Subsequently, IFN-$\lambda$ boosts the differentiation of naive B cells into plasmablasts upon activation, and the cells gain effector functions such as cytokine release (IL-6 and IL-10) and antibody production. Our study shows how IFN-$\lambda$ systematically boosts the differentiation of naive B cells into plasmablasts by enhancing the mTORC1 pathway and cell-cycle progression in activated B cells.
Cell 2020
Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients' B Cells.
Abstract
Abstract
The COVID-19 pandemic urgently needs therapeutic and prophylactic interventions. Here, we report the rapid identification of SARS-CoV-2-neutralizing antibodies by high-throughput single-cell RNA and VDJ sequencing of antigen-enriched B cells from 60 convalescent patients. From 8,558 antigen-binding IgG1+ clonotypes, 14 potent neutralizing antibodies were identified, with the most potent one, BD-368-2, exhibiting an IC50 of 1.2 and 15 ng/mL against pseudotyped and authentic SARS-CoV-2, respectively. BD-368-2 also displayed strong therapeutic and prophylactic efficacy in SARS-CoV-2-infected hACE2-transgenic mice. Additionally, the 3.8 {\AA} cryo-EM structure of a neutralizing antibody in complex with the spike-ectodomain trimer revealed the antibody's epitope overlaps with the ACE2 binding site. Moreover, we demonstrated that SARS-CoV-2-neutralizing antibodies could be directly selected based on similarities of their predicted CDR3H structures to those of SARS-CoV-neutralizing antibodies. Altogether, we showed that human neutralizing antibodies could be efficiently discovered by high-throughput single B cell sequencing in response to pandemic infectious diseases.
Cell 2019 jun
Human Antibodies that Slow Erythrocyte Invasion Potentiate Malaria-Neutralizing Antibodies.
Abstract
Abstract
The Plasmodium falciparum reticulocyte-binding protein homolog 5 (PfRH5) is the leading target for next-generation vaccines against the disease-causing blood-stage of malaria. However, little is known about how human antibodies confer functional immunity against this antigen. We isolated a panel of human monoclonal antibodies (mAbs) against PfRH5 from peripheral blood B cells from vaccinees in the first clinical trial of a PfRH5-based vaccine. We identified a subset of mAbs with neutralizing activity that bind to three distinct sites and another subset of mAbs that are non-functional, or even antagonistic to neutralizing antibodies. We also identify the epitope of a novel group of non-neutralizing antibodies that significantly reduce the speed of red blood cell invasion by the merozoite, thereby potentiating the effect of all neutralizing PfRH5 antibodies as well as synergizing with antibodies targeting other malaria invasion proteins. Our results provide a roadmap for structure-guided vaccine development to maximize antibody efficacy against blood-stage malaria.
Journal of immunology (Baltimore, Md. : 1950) 2018 NOV
Nonredundant Roles of IL-21 and IL-4 in the Phased Initiation of Germinal Center B Cells and Subsequent Self-Renewal Transitions.
Abstract
Abstract
We examined the unique contributions of the cytokines IL-21 and IL-4 on germinal center (GC) B cell initiation and subsequent maturation in a murine model system. Similar to other reports, we found T follicular helper cell expression of IL-21 begins prior to T follicular helper cell migration into the B cell follicle and precedes that of IL-4. Consistent with this timing, IL-21 signaling has a greater influence on the perifollicular pre-GC B cell transition to the intrafollicular stage. Notably, Bcl6hi B cells can form in the combined absence of IL-21R- and STAT6-derived signals; however, these nascent GC B cells cease to proliferate and are more prone to apoptosis. When B cells lack either IL-21R or STAT6, aberrant GCs form atypical centroblasts and centrocytes that differ in their phenotypic maturation and costimulatory molecule expression. Thus, IL-4 and IL-21 play nonredundant roles in the phased progression of GC B cell development that can initiate in the combined absence of these cytokine signals.
Science immunology 2018 NOV
Intrinsic properties of human germinal center B cells set antigen affinity thresholds.
Abstract
Abstract
Protective antibody responses to vaccination or infection depend on affinity maturation, a process by which high-affinity germinal center (GC) B cells are selected on the basis of their ability to bind, gather, and present antigen to T follicular helper (Tfh) cells. Here, we show that human GC B cells have intrinsically higher-affinity thresholds for both B cell antigen receptor (BCR) signaling and antigen gathering as compared with na{\{i}}ve B cells and that these functions are mediated by distinct cellular structures and pathways that ultimately lead to antigen affinity- and Tfh cell-dependent differentiation to plasma cells. GC B cells bound antigen through highly dynamic actin- and ezrin-rich pod-like structures that concentrated BCRs. The behavior of these structures was dictated by the intrinsic antigen affinity thresholds of GC B cells. Low-affinity antigens triggered continuous engagement and disengagement of membrane-associated antigens whereas high-affinity antigens induced stable synapse formation. The pod-like structures also mediated affinity-dependent antigen internalization by unconventional pathways distinct from those of na{\"{i}}ve B cells. Thus intrinsic properties of human GC B cells set thresholds for affinity selection."""
Nature communications 2016 OCT
Loss of immune tolerance to IL-2 in type 1 diabetes.
Abstract
Abstract
Type 1 diabetes (T1D) is characterized by a chronic, progressive autoimmune attack against pancreas-specific antigens, effecting the destruction of insulin-producing β-cells. Here we show interleukin-2 (IL-2) is a non-pancreatic autoimmune target in T1D. Anti-IL-2 autoantibodies, as well as T cells specific for a single orthologous epitope of IL-2, are present in the peripheral blood of non-obese diabetic (NOD) mice and patients with T1D. In NOD mice, the generation of anti-IL-2 autoantibodies is genetically determined and their titre increases with age and disease onset. In T1D patients, circulating IgG memory B cells specific for IL-2 or insulin are present at similar frequencies. Anti-IL-2 autoantibodies cloned from T1D patients demonstrate clonality, a high degree of somatic hypermutation and nanomolar affinities, indicating a germinal centre origin and underscoring the synergy between cognate autoreactive T and B cells leading to defective immune tolerance.