EasySep™ Direct Human Pan-Granulocyte Isolation Kit
Immunomagnetic negative selection from whole blood kit
概要
The EasySep™ Direct Human Pan-Granulocyte Isolation Kit isolates functional, highly purified neutrophils, eosinophils, and basophils directly from human whole blood by immunomagnetic negative selection. No lysis, density gradient centrifugation, or other processing steps are required and isolated cells are immediately available for flow cytometry, functional assays, culture, and other downstream applications.
Advantages
• > 99.9% RBC depletion without the need for density gradient centrifugation, sedimentation or lysis
• Up to 99% purity of isolated cells
• Fast, easy-to-use and column-free
• Isolated cells are untouched
• Up to 99% purity of isolated cells
• Fast, easy-to-use and column-free
• Isolated cells are untouched
Components
- EasySep™ Direct Human Pan-Granulocyte Isolation Kit (Catalog #19659)
- EasySep™ Direct Human Pan-Granulocyte Isolation Cocktail, 2 x 2.5 mL
- EasySep™ Direct RapidSpheres™, 4 x 2.5 mL
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000), or
• “The Big Easy” EasySep™ Magnet (Catalog #18001), or
• Easy 50 EasySep™ Magnet (Catalog #18002), or
• EasyEights™ EasySep™ Magnet (Catalog #18103)
Subtype
Cell Isolation Kits
Cell Type
Granulocytes and Subsets
Species
Human
Sample Source
Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Direct Human Pan-Granulocyte Isolation Kit | 19659 | All | English |
| Safety Data Sheet | EasySep™ Direct Human Pan-Granulocyte Isolation Kit | 19659 | All | English |
数据及文献
Data
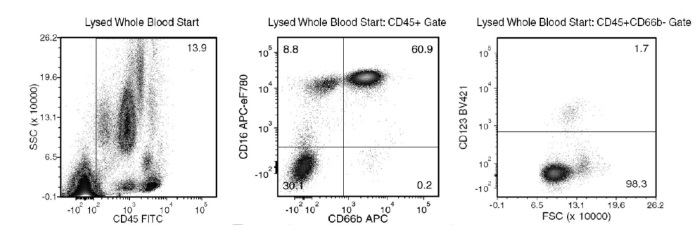
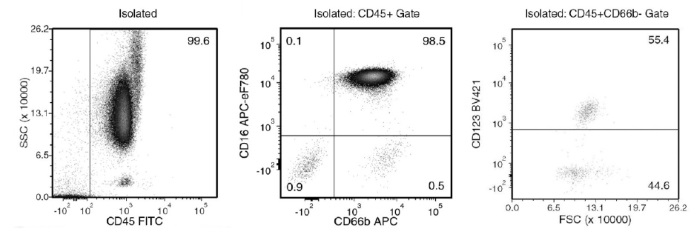
Figure 1. Typical EasySep™ Direct Human Pan-Granulocyte Isolation Profile
Starting with human whole blood from normal healthy donors, the typical pan-granulocyte (neutrophil [CD66b+CD16+], eosinophil [CD66b+CD16-] and basophil [CD66b-CD123+]) content of the non-lysed final isolated fraction is 98.4 ± 1.5% (gated on CD45). In the above example, the pan-granulocyte content of the lysed whole blood start sample and the non-lysed final isolated fraction is 61.8% and 99.6% (gated on CD45), respectively.
Publications (2)
Nature 2019 apr
Nuclear positioning facilitates amoeboid migration along the path of least resistance.
Abstract
Abstract
During metazoan development, immune surveillance and cancer dissemination, cells migrate in complex three-dimensional microenvironments1-3. These spaces are crowded by cells and extracellular matrix, generating mazes with differently sized gaps that are typically smaller than the diameter of the migrating cell4,5. Most mesenchymal and epithelial cells and some-but not all-cancer cells actively generate their migratory path using pericellular tissue proteolysis6. By contrast, amoeboid cells such as leukocytes use non-destructive strategies of locomotion7, raising the question how these extremely fast cells navigate through dense tissues. Here we reveal that leukocytes sample their immediate vicinity for large pore sizes, and are thereby able to choose the path of least resistance. This allows them to circumnavigate local obstacles while effectively following global directional cues such as chemotactic gradients. Pore-size discrimination is facilitated by frontward positioning of the nucleus, which enables the cells to use their bulkiest compartment as a mechanical gauge. Once the nucleus and the closely associated microtubule organizing centre pass the largest pore, cytoplasmic protrusions still lingering in smaller pores are retracted. These retractions are coordinated by dynamic microtubules; when microtubules are disrupted, migrating cells lose coherence and frequently fragment into migratory cytoplasmic pieces. As nuclear positioning in front of the microtubule organizing centre is a typical feature of amoeboid migration, our findings link the fundamental organization of cellular polarity to the strategy of locomotion.
Nature communications 2019
High-throughput single-cell rheology in complex samples by dynamic real-time deformability cytometry.
Abstract
Abstract
In life sciences, the material properties of suspended cells have attained significance close to that of fluorescent markers but with the advantage of label-free and unbiased sample characterization. Until recently, cell rheological measurements were either limited by acquisition throughput, excessive post processing, or low-throughput real-time analysis. Real-time deformability cytometry expanded the application of mechanical cell assays to fast on-the-fly phenotyping of large sample sizes, but has been restricted to single material parameters as the Young's modulus. Here, we introduce dynamic real-time deformability cytometry for comprehensive cell rheological measurements at up to 100 cells per second. Utilizing Fourier decomposition, our microfluidic method is able to disentangle cell response to complex hydrodynamic stress distributions and to determine viscoelastic parameters independent of cell shape. We demonstrate the application of our technology for peripheral blood cells in whole blood samples including the discrimination of B- and CD4+ T-lymphocytes by cell rheological properties.