EasySep™ Direct Human Neutrophil Isolation Kit
Immunomagnetic isolation of neutrophils directly from whole blood
概要
The EasySep™ Direct Human Neutrophil Isolation Kit isolates functional, highly purified neutrophils directly from human whole blood by immunomagnetic negative selection. No lysis, density gradient centrifugation or other processing steps are required and isolated cells are immediately available for flow cytometry, functional assays, culture, and other downstream applications.
This product replaces the EasySep™ Human Neutrophil Isolation Kit (Catalog #19257) for even faster cell isolations.
This product replaces the EasySep™ Human Neutrophil Isolation Kit (Catalog #19257) for even faster cell isolations.
Advantages
• > 99.9% RBC depletion without the need for density gradient centrifugation, sedimentation, or lysis
• Up to 99% purity of isolated cells
• Fast, easy-to-use and column-free
• Isolated cells are untouched
• Up to 99% purity of isolated cells
• Fast, easy-to-use and column-free
• Isolated cells are untouched
Components
- EasySep™ Direct Human Neutrophil Isolation Kit (Catalog #19666)
- EasySep™ Direct Human Neutrophil Isolation Cocktail, 2 x 2.5 mL
- EasySep™ Direct RapidSpheres™, 4 x 2.5 mL
- RoboSep™ Human Neutrophil Isolation Kit (Catalog #100-0404)
- EasySep™ Direct Human Neutrophil Isolation Cocktail, 2 x 2.5 mL
- EasySep™ Direct RapidSpheres™, 4 x 2.5 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• Easy 50 EasySep™ Magnet (Catalog #18002)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Granulocytes and Subsets
Species
Human
Sample Source
Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology, Infectious Diseases
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Direct Human Neutrophil Isolation Kit | 19666 | All | English |
| Product Information Sheet | RoboSep™ Human Neutrophil Isolation Kit | 100-0404 | All | English |
| Safety Data Sheet 1 | EasySep™ Direct Human Neutrophil Isolation Kit | 19666 | All | English |
| Safety Data Sheet 2 | EasySep™ Direct Human Neutrophil Isolation Kit | 19666 | All | English |
数据及文献
Data
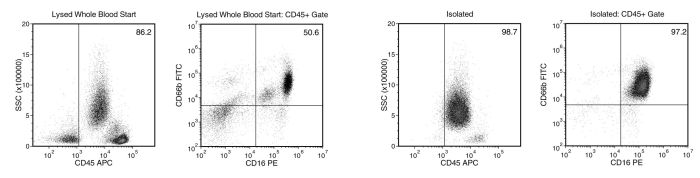
Figure 1. Typical EasySep™ Direct Human Neutrophil Isolation Profile
Starting with human whole blood from normal healthy donors, the typical neutrophil (CD66b+CD16+) content of the non-lysed final isolated fraction Is 97.3 ± 1.4% (gated on CD45) or 94.0 ± 3.7% (not gated on CD45). In the example above, the neutrophil (CD66b+CD16+) content of the lysed whole blood start sample and the non-lysed final isolated fraction is 50.6% and 97.2% (gated on CD45), respectively, or 43.6% and 95.9% (not gated on CD45), respectively. The starting frequency of neutrophils in the non-lysed whole blood start sample above is 0.04% (data not shown).
Publications (12)
Cell reports 2020 jan
The Regulatory Proteins Rtg1/3 Govern Sphingolipid Homeostasis in the Human-Associated Yeast Candida albicans.
Abstract
Abstract
Integrating nutrient sensing with the synthesis of complex molecules is a central feature of metabolism. Yet the regulatory mechanisms underlying such integration are often unknown. Here, we establish that the transcription regulators Rtg1/3 are key determinants of sphingolipid homeostasis in the human fungal pathogen Candida albicans. Quantitative analysis of the C. albicans lipidome reveals Rtg1/3-dependent alterations in all complex sphingolipids and their precursors, ceramides. Mutations in the regulators render the fungus susceptible to myriocin, a sphingolipid synthesis inhibitor. Rtg1/3 exert control on the expression of several enzymes involved in the synthesis of sphingolipids' building blocks, and the regulators are activated upon engulfment of C. albicans cells by human neutrophils. We demonstrate that Rtg1p and Rtg3p are regulated at two levels, one in response to sphingolipids and the other by the nutrient sensor TOR. Our findings, therefore, indicate that the Rtg1/3 system integrates nutrient sensing into the synthesis of complex lipids.
Blood advances 2020 jan
N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm.
Abstract
Abstract
Thrombosis is a major cause of mortality in patients with myeloproliferative neoplasms (MPNs), though there is currently little to offer patients with MPN beyond aspirin and cytoreductive therapies such as hydroxyurea for primary prevention. Thrombogenesis in MPN involves multiple cellular mechanisms, including platelet activation and neutrophil-extracellular trap formation; therefore, an antithrombotic agent that targets one or more of these processes would be of therapeutic benefit in MPN. Here, we treated the JAK2V617F knockin mouse model of polycythemia vera with N-acetylcysteine (NAC), a sulfhydryl-containing compound with broad effects on glutathione replenishment, free radical scavenging, and reducing disulfide bonds, to investigate its antithrombotic effects in the context of MPN. Strikingly, NAC treatment extended the lifespan of JAK2V617F mice without impacting blood counts or splenomegaly. Using an acute pulmonary thrombosis model in vivo, we found that NAC reduced thrombus formation to a similar extent as the irreversible platelet inhibitor aspirin. In vitro analysis of platelet activation revealed that NAC reduced thrombin-induced platelet-leukocyte aggregate formation in JAK2V617F mice. Furthermore, NAC reduced neutrophil extracellular trap formation in primary human neutrophils from patients with MPN as well as healthy controls. These results provide evidence that N-acetylcysteine inhibits thrombosis in JAK2V617F mice and provide a pre-clinical rationale for investigating NAC as a therapeutic to reduce thrombotic risk in MPN.
Blood 2020
Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.
Abstract
Abstract
COVID-19 affects millions of patients worldwide, with clinical presentation ranging from isolated thrombosis to acute respiratory distress syndrome (ARDS) requiring ventilator support. Neutrophil extracellular traps (NETs) originate from decondensed chromatin released to immobilize pathogens, and they can trigger immunothrombosis. We studied the connection between NETs and COVID-19 severity and progression. We conducted a prospective cohort study of COVID-19 patients (n = 33) and age- and sex-matched controls (n = 17). We measured plasma myeloperoxidase (MPO)-DNA complexes (NETs), platelet factor 4, RANTES, and selected cytokines. Three COVID-19 lung autopsies were examined for NETs and platelet involvement. We assessed NET formation ex vivo in COVID-19 neutrophils and in healthy neutrophils incubated with COVID-19 plasma. We also tested the ability of neonatal NET-inhibitory factor (nNIF) to block NET formation induced by COVID-19 plasma. Plasma MPO-DNA complexes increased in COVID-19, with intubation (P {\textless} .0001) and death (P {\textless} .0005) as outcome. Illness severity correlated directly with plasma MPO-DNA complexes (P = .0360), whereas Pao2/fraction of inspired oxygen correlated inversely (P = .0340). Soluble and cellular factors triggering NETs were significantly increased in COVID-19, and pulmonary autopsies confirmed NET-containing microthrombi with neutrophil-platelet infiltration. Finally, COVID-19 neutrophils ex vivo displayed excessive NETs at baseline, and COVID-19 plasma triggered NET formation, which was blocked by nNIF. Thus, NETs triggering immunothrombosis may, in part, explain the prothrombotic clinical presentations in COVID-19, and NETs may represent targets for therapeutic intervention.
Cell communication and signaling : CCS 2019 dec
Cholesterol restricts lymphotoxin $\beta$ receptor-triggered NF-$\kappa$B signaling.
Abstract
Abstract
BACKGROUND Lymphotoxin $\beta$ receptor (LT$\beta$R) plays important roles in the development of the immune system and immune response. At the cellular level, ligand-bound LT$\beta$R activates the pro-inflammatory NF-$\kappa$B pathway but the detailed mechanisms regulating its signaling remain unknown. Understanding them is of high importance since LT$\beta$R and its ligands are promising therapeutic targets. Here, we studied the consequences of perturbed cellular cholesterol content on LT$\beta$R-induced NF-$\kappa$B signaling. METHODS To modulate cholesterol availability and/or level in lung carcinoma A549 and H2228, and endothelial HUVEC cells different treatment regimens with filipin, methyl-$\beta$-cyclodextrin and simvastatin were applied. LT$\beta$R localization was studied by confocal microscopy. The activity of LT$\beta$R-induced NF-$\kappa$B pathway was assessed by measuring the levels of NF-$\kappa$B pathway inhibitor I$\kappa$B$\alpha$ and phosphorylation of RelA transcription factor by Western blotting. The NF-$\kappa$B transcriptional response, production of chemokines and adhesion molecules were examined by qRT-PCR, ELISA, and Western blotting, respectively. Adherence of different types of primary immune cells to epithelial A549 cells and endothelial HUVECs was measured fluorometrically. Interactions of LT$\beta$R with its protein partners were investigated by immunoprecipitation. RESULTS We showed that filipin-mediated sequestration of cholesterol or its depletion from the plasma membrane with methyl-$\beta$-cyclodextrin impaired LT$\beta$R internalization and potentiated LT$\beta$R-dependent activation of the canonical branch of the NF-$\kappa$B pathway. The latter was manifested by enhanced degradation of I$\kappa$B$\alpha$ inhibitor, elevated RelA phosphorylation, substantial increase in the expression of NF-$\kappa$B target genes encoding, among others, cytokines and adhesion molecules known to play important roles in immune response. It was followed by robust secretion of CXCL8 and upregulation of ICAM1, that favored the adhesion of immune cells (NK and T cells, neutrophils) to A549 cells and HUVECs. Mechanistically, we showed that cholesterol depletion stabilized interactions of ligand-stimulated LT$\beta$R with modified forms of TRAF2 and NEMO proteins. CONCLUSIONS Our results showed that the reduction of the plasma membrane content of cholesterol or its sequestration strongly potentiated signaling outcome initiated by LT$\beta$R. Thus, drugs modulating cholesterol levels could potentially improve efficacy of LT$\beta$R-based therapies. Video abstract.
Nature communications 2018 NOV
Cis interaction between sialylated Fc$\gamma$RIIA and the $\alpha$I-domain of Mac-1 limits antibody-mediated neutrophil recruitment.
Abstract
Abstract
Vascular-deposited IgG immune complexes promote neutrophil recruitment, but how this process is regulated is still unclear. Here we show that the CD18 integrin Mac-1, in its bent state, interacts with the IgG receptor Fc$\gamma$RIIA in cis to reduce the affinity of Fc$\gamma$RIIA for IgG and inhibit Fc$\gamma$RIIA-mediated neutrophil recruitment under flow. The Mac-1 rs1143679 lupus-risk variant reverses Mac-1 inhibition of Fc$\gamma$RIIA, as does a Mac-1 ligand and a mutation in Mac-1's ligand binding $\alpha$I-domain. Sialylated complex glycans on Fc$\gamma$RIIA interact with the $\alpha$I-domain via divalent cations, and this interaction is required for Fc$\gamma$RIIA inhibition by Mac-1. Human neutrophils deficient in CD18 integrins exhibit augmented Fc$\gamma$RIIA-dependent recruitment to IgG-coated endothelium. In mice, CD18 integrins on neutrophils dampen IgG-mediated neutrophil accumulation in the kidney. In summary, cis interaction between sialylated Fc$\gamma$RIIA and the $\alpha$I-domain of Mac-1 alters the threshold for IgG-mediated neutrophil recruitment. A disruption of this interaction may increase neutrophil influx in autoimmune diseases.
The Biochemical journal 2018 JAN
Interrogating Parkinson's disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils.
Abstract
Abstract
There is compelling evidence for the role of the leucine-rich repeat kinase 2 (LRRK2) and in particular its kinase function in Parkinson's disease. Orally bioavailable, brain penetrant and potent LRRK2 kinase inhibitors are in the later stages of clinical development. Here, we describe a facile and robust assay to quantify LRRK2 kinase pathway activity by measuring LRRK2-mediated phosphorylation of Rab10 in human peripheral blood neutrophils. We use the selective MJFF-pRab10 monoclonal antibody recognising the Rab10 Thr73 phospho-epitope that is phosphorylated by LRRK2. We highlight the feasibility and practicability of using our assay in the clinical setting by studying a few patients with G2019S LRRK2 associated and sporadic Parkinson's as well as healthy controls. We suggest that peripheral blood neutrophils are a valuable resource for LRRK2 research and should be considered for inclusion in Parkinson's bio-repository collections as they are abundant, homogenous and express relatively high levels of LRRK2 as well as Rab10. In contrast, the widely used peripheral blood mononuclear cells are heterogeneous and only a minority of cells (monocytes and contaminating neutrophils) express LRRK2. While our LRRK2 kinase pathway assay could assist in patient stratification based on LRRK2 kinase activity, we envision that it may find greater utility in pharmacodynamic and target engagement studies in future LRRK2 inhibitor trials.