StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit
Immunomagnetic column-based negative selection kit
概要
The StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit is designed to isolate hematopoietic progenitor cells from fresh or previously frozen mobilized peripheral blood, cord blood or bone marrow by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, glycophorin A and dextran-coated magnetic particles. Labeled cells are separated using StemSep™ columns and magnets.
Components
- StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit (Catalog #14056)
- StemSep™ Human Hematopoietic Progenitor Cell Enrichment Cocktail, 2 mL
- StemSep™ Magnetic Colloid, 1.5 mL
- StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit (Catalog #14066)
- StemSep™ Human Hematopoietic Progenitor Cell Enrichment Cocktail, 5 x 2 mL
- StemSep™ Magnetic Colloid, 4 x 1.5 mL
Magnet Compatibility
• StemSep™ Magnet (Catalog #11030, 11050, 11060 11070) or a magnet with the strength of at least 0.5 Tesla
• StemSep™ Negative Selection Columns
Subtype
Cell Isolation Kits
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Human
Sample Source
Bone Marrow
Selection Method
Negative
Application
Cell Isolation
Brand
StemSep
Area of Interest
Immunology, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit | 14056, 14066 | All | English |
| Manual | StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit | 14056 | All | English |
| Safety Data Sheet 1 | StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit | 14056, 14066 | All | English |
| Safety Data Sheet 2 | StemSep™ Human Hematopoietic Progenitor Cell Enrichment Kit | 14056 | All | English |
数据及文献
Data
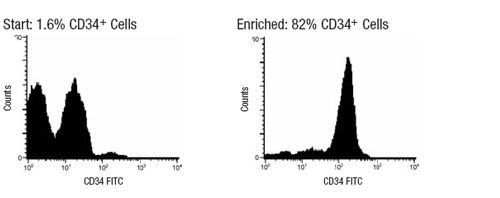
Figure 1. Typical StemSep™ Hematopoietic Progenitor Cell Profile
The frequency of CD34+ cells in the enriched fraction is typically: 74 - 88% (mobilized peripheral blood), 30 - 50% (bone marrow), or 45 - 61% (cord blood).
Publications (5)
Blood 2010 JAN
The AC133+CD38-, but not the rhodamine-low, phenotype tracks LTC-IC and SRC function in human cord blood ex vivo expansion cultures.
Abstract
Abstract
Phenotypic markers associated with human hematopoietic stem cells (HSCs) were developed and validated using uncultured cells. Because phenotype and function can be dissociated during culture, better markers to prospectively track and isolate HSCs in ex vivo cultures could be instrumental in advancing HSC-based therapies. Using an expansion system previously shown to increase hematopoietic progenitors and SCID-repopulating cells (SRCs), we demonstrated that the rhodamine-low phenotype was lost, whereas AC133 expression was retained throughout culture. Furthermore, the AC133(+)CD38(-) subpopulation was significantly enriched in long-term culture-initiating cells (LTC-IC) and SRCs after culture. Preculture and postculture analysis of total nucleated cell and LTC-IC number, and limiting dilution analysis in NOD/SCID mice, showed a 43-fold expansion of the AC133(+)CD38(-) subpopulation that corresponded to a 7.3-fold and 4.4-fold expansion of LTC-ICs and SRCs in this subpopulation, respectively. Thus, AC133(+)CD38(-) is an improved marker that tracks and enriches for LTC-IC and SRC in ex vivo cultures.
Blood 2010 DEC
Progenitor cell dose determines the pace and completeness of engraftment in a xenograft model for cord blood transplantation.
Abstract
Abstract
Two critical concerns in clinical cord blood transplantation are the initial time to engraftment and the subsequent restoration of immune function. These studies measured the impact of progenitor cell dose on both the pace and strength of hematopoietic reconstitution by transplanting nonobese diabetic/severe combined immunodeficiency/interleukin-2 receptor-gamma-null (NSγ) mice with lineage-depleted aldehyde dehydrogenase-bright CD34(+) human cord blood progenitors. The progress of each transplant was monitored over an extended time course by repeatedly analyzing the peripheral blood for human hematopoietic cells. In vivo human hematopoietic development was complete. After long-term transplantation assays (≥ 19 weeks), human T-cell development was documented within multiple tissues in 16 of 32 NSγ mice. Human T-cell differentiation was active within NSγ thymuses, as documented by the presence of CD4(+) CD8(+) T-cell progenitors as well as T-cell receptor excision circles. It is important to note that although myeloid and B-cell engraftment was detected as early as 4 weeks after transplantation, human T-cell development was exclusively late onset. High progenitor cell doses were associated with a robust human hematopoietic chimerism that accelerated both initial time to engraftment and subsequent T-cell development. At lower progenitor cell doses, the chimerism was weak and the human hematopoietic lineage development was frequently incomplete.
Blood 2009 OCT
Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells.
Abstract
Abstract
Off-patent drugs with previously unrecognized anticancer activity could be rapidly repurposed for this new indication. To identify such compounds, we conducted 2 independent cell-based chemical screens and identified the antimicrobial ciclopirox olamine (CPX) in both screens. CPX decreased cell growth and viability of malignant leukemia, myeloma, and solid tumor cell lines as well as primary AML patient samples at low-micromolar concentrations that appear pharmacologically achievable. Furthermore, oral CPX decreased tumor weight and volume in 3 mouse models of leukemia by up to 65% compared with control without evidence of weight loss or gross organ toxicity. In addition, oral CPX prevented the engraftment of primary AML cells in nonobese diabetic/severe combined immunodeficiency mouse models, thereby establishing its ability to target leukemia stem cells. Mechanistically, CPX bound intracellular iron, and this intracellular iron chelation was functionally important for its cytotoxicity. By electron paramagnetic resonance, CPX inhibited the iron-dependent enzyme ribonucleotide reductase at concentrations associated with cell death. Thus, in summary, CPX has previously unrecognized anticancer activity at concentrations that are pharmacologically achievable. Therefore, CPX could be rapidly repurposed for the treatment of malignancies, including leukemia and myeloma.
Journal of immunological methods 2008 MAR
Increased production of megakaryocytes near purity from cord blood CD34+ cells using a short two-phase culture system.
Abstract
Abstract
Expansion of hematopoietic progenitor cells (HPC) ex vivo remains an important focus in fundamental and clinical research. The aim of this study was to determine whether the implementation of such expansion phase in a two-phase culture strategy prior to the induction of megakaryocyte (Mk) differentiation would increase the yield of Mks produced in cultures. Toward this end, we first characterized the functional properties of five cytokine cocktails to be tested in the expansion phase on the growth and differentiation kinetics of CD34+-enriched cells, and on their capacity to expand clonogenic progenitors in cultures. Three of these cocktails were chosen based on their reported ability to induce HPC expansion ex vivo, while the other two represented new cytokine combinations. These analyses revealed that none of the cocktails tested could prevent the differentiation of CD34+ cells and the rapid expansion of lineage-positive cells. Hence, we sought to determine the optimum length of time for the expansion phase that would lead to the best final Mk yields. Despite greater expansion of CD34+ cells and overall cell growth with a longer expansion phase, the optimal length for the expansion phase that provided greater Mk yield at near maximal purity was found to be 5 days. Under such settings, two functionally divergent cocktails were found to significantly increase the final yield of Mks. Surprisingly, these cocktails were either deprived of thrombopoietin or of stem cell factor, two cytokines known to favor megakaryopoiesis and HPC expansion, respectively. Based on these results, a short resource-efficient two-phase culture protocol for the production of Mks near purity (textgreater95%) from human CD34+ CB cells has been established.
Stem cells and development 2008 JUN
Characterization of the effects and potential mechanisms leading to increased megakaryocytic differentiation under mild hyperthermia.
Abstract
Abstract
The physical culture parameters have important influences on the proliferation and differentiation fate of hematopoietic stem cells. Recently, we have demonstrated that CD34+ cord blood (CB) cells undergo accelerated and increased megakaryocyte (Mk) differentiation when incubated under mild hyperthermic conditions (i.e., 39 degrees C). In this study, we investigated in detail the impacts of mild hyperthermia on Mk differentiation and maturation, and explored potential mechanisms responsible for these phenomena. Our results demonstrate that the qualitative and quantitative effects on Mk differentiation at 39 degrees C appear rapidly within 7 days, and that early transient culture at 39 degrees C led to even greater Mk yields (ptextless0.03). Surprisingly, cell viability was only found to be significantly reduced in the early stages of culture, suggesting that CB cells are able with time to acclimatize themselves to 39 degrees C. Although mild hyperthermia accelerated differentiation and maturation of CB-derived Mks, it failed to promote their polyploidization further but rather led to a small reduction in the proportion of polyploid Mks (p=0.01). Conversely, gene arrays analysis demonstrated that Mks derived at 39 degrees C have a normal gene expression program consistent with an advanced maturation state. Finally, two independent mechanisms that could account for the accelerated Mk differentiation were investigated. Our results suggest that the accelerated and increased Mk differentiation induced by mild hyperthermia is not mediated by cell-secreted factors but could perhaps be mediated by the increased expression of Mk transcription factors.




