SepMate™-50 (IVD)
Tube for density gradient centrifugation for in vitro diagnostic (IVD) applications
概要
SepMate™ is now manufactured under cGMP and registered as an In Vitro Diagnostic (IVD) device in Australia, Canada, Europe and USA only. In China SepMate™ is considered a non-medical device by the China Food and Drug Administration (CFDA), and should therefore be used as general laboratory equipment. The end user is responsible for determining whether the product is suitable for their specific application. Due to these changes, users in Australia, Canada, Europe, USA and China should, from now on, order SepMate™-15 (IVD) (Catalog #85415 and #85420) and SepMate™-50 (IVD) (Catalog #85450 and #85460). Users in all other countries please refer to SepMate™-15 (RUO) (Catalog #86415) and SepMate™-50 (RUO) (Catalog #86450). Changes to SepMate™ have no impact on the product composition, performance or protocol.
SepMate™ is a tube that facilitates the isolation of PBMCs or specific cell types by density gradient centrifugation. SepMate™ tubes contain an insert that provides a barrier between the density gradient medium and blood. SepMate™ eliminates the need for careful layering of blood onto the density gradient medium, and allows for fast and easy harvesting of the isolated mononuclear cells with a simple pour. SepMate™ is also compatible with RosetteSep™ enrichment cocktails, allowing isolation of specific cell types in less than 30 minutes.
SepMate™ is a tube that facilitates the isolation of PBMCs or specific cell types by density gradient centrifugation. SepMate™ tubes contain an insert that provides a barrier between the density gradient medium and blood. SepMate™ eliminates the need for careful layering of blood onto the density gradient medium, and allows for fast and easy harvesting of the isolated mononuclear cells with a simple pour. SepMate™ is also compatible with RosetteSep™ enrichment cocktails, allowing isolation of specific cell types in less than 30 minutes.
Advantages
• Eliminates the need for carefully layering blood over the density gradient medium (e.g. Lymphoprep™, etc.)
• Reduces total centrifuge time to 10 minutes with the brake on for fresh samples
• Allows fast and easy harvesting of the isolated mononuclear cells by simply pouring off the supernatant
• Can be combined with RosetteSep™ enrichment cocktails to isolate specific cell types in just 30 minutes
• Reduces total centrifuge time to 10 minutes with the brake on for fresh samples
• Allows fast and easy harvesting of the isolated mononuclear cells by simply pouring off the supernatant
• Can be combined with RosetteSep™ enrichment cocktails to isolate specific cell types in just 30 minutes
Components
- SepMate™-50 (IVD), 100 Tubes (Catalog #85450)
- Dispenser box containing 4 bags, 25 Tubes/Bag
- SepMate™-50 (IVD), 500 Tubes (Catalog #85460)
- Dispenser box containing 4 bags, 25 Tubes/Bag (Catalog #85450) x 5
Contains
Polypropylene tube containing an insert
Subtype
Centrifugation Tubes
Cell Type
B Cells, Dendritic Cells, Monocytes, Mononuclear Cells, NK Cells, T Cells, T Cells, CD4+, T Cells, CD8+, T Cells, Other Subsets, T Cells, Regulatory
Species
Human
Sample Source
Bone Marrow, Cord Blood, Whole Blood
Selection Method
Negative
Application
Cell Isolation, In Vitro Diagnostic
Brand
SepMate
Area of Interest
Chimerism, HLA, Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | SepMate™-50 (IVD) | 85450, 85460 | All | English |
数据及文献
Data
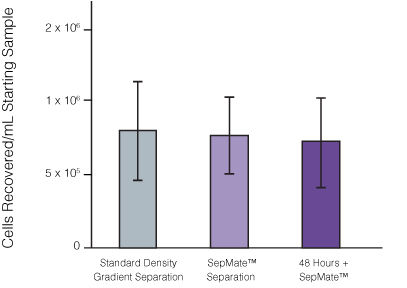
Figure 1. Recovery of mononuclear cells (MNCs) from peripheral blood using SepMate™-50 versus standard density gradient centrifiguation. Recovery of MNCs from fresh and 48-hour post blood draw enriched by density gradient centrifugation with SepMate™ (purple) or without (grey). There was no significant difference in the recovery of MNCS with and without SepMate™.
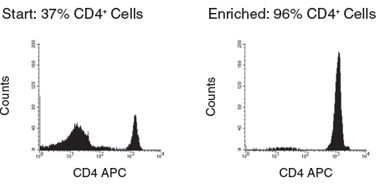
Figure 2. Human CD4+ T Cell Isolation using SepMate™-50 and RosetteSep™ Human CD4+ T Cell Enrichment Cocktail
Publications (44)
The Journal of experimental medicine 2020 oct
Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients.
Abstract
Abstract
Cellular immunotherapeutics targeting the human papillomavirus (HPV)-16 E6 and E7 proteins have achieved limited success in HPV-positive oropharyngeal cancer (OPC). Here we have conducted proteome-wide profiling of HPV-16-specific T cell responses in a cohort of 66 patients with HPV-associated OPC and 22 healthy individuals. Unexpectedly, HPV-specific T cell responses from OPC patients were not constrained to the E6 and E7 antigens; they also recognized E1, E2, E4, E5, and L1 proteins as dominant targets for virus-specific CD8+ and CD4+ T cells. Multivariate analysis incorporating tumor staging, treatment status, and smoking history revealed that treatment status had the most significant impact on HPV-specific CD8+ and CD4+ T cell immunity. Specifically, the breadth and overall strength of HPV-specific T cell responses were significantly higher before the commencement of curative therapy than after therapy. These data provide the first glimpse of the overall human T cell response to HPV in a clinical setting and offer groundbreaking insight into future development of cellular immunotherapies for HPV-associated OPC patients.
Viruses 2020 jun
The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages' Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis.
Abstract
Abstract
Bovine viral diarrhea virus (BVDV) is an important viral disease of cattle that causes immune dysfunction. Macrophages are the key cells for the initiation of the innate immunity and play an important role in viral pathogenesis. In this in vitro study, we studied the effect of the supernatant of BVDV-infected macrophage on immune dysfunction. We infected bovine monocyte-derived macrophages (MDM) with high or low virulence strains of BVDV. The supernatant recovered from BVDV-infected MDM was used to examine the functional activity and surface marker expression of normal macrophages as well as lymphocyte apoptosis. Supernatants from the highly virulent 1373-infected MDM reduced phagocytosis, bactericidal activity and downregulated MHC II and CD14 expression of macrophages. Supernatants from 1373-infected MDM induced apoptosis in MDBK cells, lymphocytes or BL-3 cells. By protein electrophoresis, several protein bands were unique for high-virulence, 1373-infected MDM supernatant. There was no significant difference in the apoptosis-related cytokine mRNA (IL-1beta, IL-6 and TNF-a) of infected MDM. These data suggest that BVDV has an indirect negative effect on macrophage functions that is strain-specific. Further studies are required to determine the identity and mechanism of action of these virulence factors present in the supernatant of the infected macrophages.
Scientific reports 2020 jun
Vaccination of koalas during antibiotic treatment for Chlamydia-induced cystitis induces an improved antibody response to Chlamydia pecorum.
Abstract
Abstract
Chlamydia infection and disease are endemic in free-ranging koalas. Antibiotics remain the front line treatment for Chlamydia in koalas, despite their rates of treatment failure and adverse gut dysbiosis outcomes. A Chlamydia vaccine for koalas has shown promise for replacing antibiotic treatment in mild ocular Chlamydia disease. In more severe disease presentations that require antibiotic intervention, the effect of vaccinating during antibiotic use is not currently known. This study investigated whether a productive immune response could be induced by vaccinating koalas during antibiotic treatment for Chlamydia-induced cystitis. Plasma IgG antibody levels against the C. pecorum major outer membrane protein (MOMP) dropped during antibiotic treatment in both vaccinated and unvaccinated koalas. Post-treatment, IgG levels recovered. The IgG antibodies from naturally-infected, vaccinated koalas recognised a greater proportion of the MOMP protein compared to their naturally-infected, unvaccinated counterparts. Furthermore, peripheral blood mononuclear cell gene expression revealed an up-regulation in genes related to neutrophil degranulation in vaccinated koalas during the first month post-vaccination. These findings show that vaccination of koalas while they are being treated with antibiotics for cystitis can result in the generation of a productive immune response, in the form of increased and expanded IgG production and host response through neutrophil degranulation.
Science (New York, N.Y.) 2020 jul
Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications.
Abstract
Abstract
COVID-19 is currently a global pandemic, but human immune responses to the virus remain poorly understood. We analyzed 125 COVID-19 patients, and compared recovered to healthy individuals using high dimensional cytometry. Integrated analysis of {\~{}}200 immune and {\~{}}50 clinical features revealed activation of T cell and B cell subsets in a proportion of patients. A subgroup of patients had T cell activation characteristic of acute viral infection and plasmablast responses reaching {\textgreater}30{\%} of circulating B cells. However, another subgroup had lymphocyte activation comparable to uninfected subjects. Stable versus dynamic immunological signatures were identified and linked to trajectories of disease severity change. These analyses identified three immunotypes" associated with poor clinical trajectories versus improving health. These immunotypes may have implications for the design of therapeutics and vaccines for COVID-19."
Nature biomedical engineering 2020 jul
Classification of T-cell activation via autofluorescence lifetime imaging.
Abstract
Abstract
The function of a T cell depends on its subtype and activation state. Here, we show that imaging of the autofluorescence lifetime signals of quiescent and activated T cells can be used to classify the cells. T cells isolated from human peripheral blood and activated in culture using tetrameric antibodies against the surface ligands CD2, CD3 and CD28 showed specific activation-state-dependent patterns of autofluorescence lifetime. Logistic regression models and random forest models classified T cells according to activation state with 97-99{\%} accuracy, and according to activation state (quiescent or activated) and subtype (CD3+CD8+ or CD3+CD4+) with 97{\%} accuracy. Autofluorescence lifetime imaging can be used to non-destructively determine T-cell function.
Nature 2020 aug
Sex differences in immune responses that underlie COVID-19 disease outcomes.
Abstract
Abstract
There is increasing evidence that coronavirus disease 2019 (COVID-19) produces more severe symptoms and higher mortality among men than among women1-5. However, whether immune responses against severe acute respiratory syndrome coronavirus (SARS-CoV-2) differ between sexes, and whether such differences correlate with the sex difference in the disease course of COVID-19, is currently unknown. Here we examined sex differences in viral loads, SARS-CoV-2-specific antibody titres, plasma cytokines and blood-cell phenotyping in patients with moderate COVID-19 who had not received immunomodulatory medications. Male patients had higher plasma levels of innate immune cytokines such as IL-8 and IL-18 along with more robust induction of non-classical monocytes. By contrast, female patients had more robust T cell activation than male patients during SARS-CoV-2 infection. Notably, we found that a poor T cell response negatively correlated with patients' age and was associated with worse disease outcome in male patients, but not in female patients. By contrast, higher levels of innate immune cytokines were associated with worse disease progression in female patients, but not in male patients. These findings provide a possible explanation for the observed sex biases in COVID-19, and provide an important basis for the development of a sex-based approach to the treatment and care of male and female patients with COVID-19.