SepMate™-15 (RUO)
Tube for density gradient centrifugation
概要
SepMate™ is a tube that facilitates the isolation of PBMCs or specific cell types by density gradient centrifugation. SepMate™ tubes contain an insert that provides a barrier between the density gradient medium and blood. SepMate™ eliminates the need for careful layering of blood (or bone marrow or cord blood) onto the density gradient medium, and allows for fast and easy harvesting of the isolated mononuclear cells with a simple pour. SepMate™ is also compatible with RosetteSep™ enrichment cocktails, allowing isolation of specific cell types in less than 30 minutes.
SepMate™-15 is designed for processing samples from 0.5 to 5 mL of sample.
SepMate™-15 (RUO) (Catalog #86415 and #86420) is manufactured under cGMP and is for Research Use Only. Users in Australia, Canada, Europe and USA, please refer to SepMate™-15 (IVD) (Catalog #85415 and #85420).
SepMate™-15 is designed for processing samples from 0.5 to 5 mL of sample.
SepMate™-15 (RUO) (Catalog #86415 and #86420) is manufactured under cGMP and is for Research Use Only. Users in Australia, Canada, Europe and USA, please refer to SepMate™-15 (IVD) (Catalog #85415 and #85420).
Advantages
• Eliminates the need for carefully layering blood over the density gradient medium (e.g. Ficoll-Paque™, Lymphoprep™ )
• Reduces total centrifuge time to 10 minutes with the brake on for fresh samples
• Allows fast and easy harvesting of the isolated mononuclear cells by simply pouring off the supernatant
• Can be combined with RosetteSep™ enrichment cocktails to isolate specific cell types in just 25 minutes
• Reduces total centrifuge time to 10 minutes with the brake on for fresh samples
• Allows fast and easy harvesting of the isolated mononuclear cells by simply pouring off the supernatant
• Can be combined with RosetteSep™ enrichment cocktails to isolate specific cell types in just 25 minutes
Components
- SepMate™-15 (RUO), 100 Tubes (Catalog #86415)
- Dispenser box containing 4 bags, 25 Tubes/Bag
- SepMate™-15 (RUO), 500 Tubes (Catalog #86420)
- Dispenser box containing 4 bags, 25 Tubes/Bag (Catalog #86415) x 5
Contains
Polypropylene tube containing an insert
Subtype
Centrifugation Tubes
Cell Type
B Cells, Dendritic Cells, Monocytes, Mononuclear Cells, NK Cells, T Cells, T Cells, CD4+, T Cells, CD8+, T Cells, Other Subsets, T Cells, Regulatory
Species
Human
Sample Source
Bone Marrow, Cord Blood, Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
SepMate
Area of Interest
Chimerism, HLA, Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | SepMate™-15 (RUO) | 86415, 86420 | All | English |
数据及文献
Data
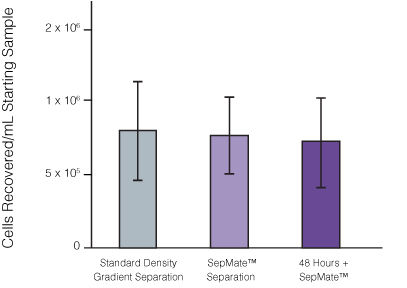
Figure 1. Recovery of mononuclear cells (MNCs) from peripheral blood using SepMate™-50 versus standard density gradient centrifiguation. Recovery of MNCs from fresh and 48-hour post blood draw enriched by density gradient centrifugation with SepMate™ (purple) or without (grey). There was no significant difference in the recovery of MNCS with and without SepMate™.
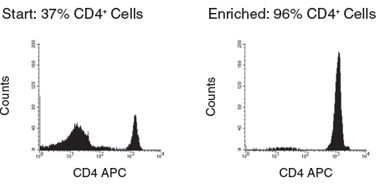
Figure 2. Human CD4+ T Cell Isolation using SepMate™-50 and RosetteSep™ Human CD4+ T Cell Enrichment Cocktail
Publications (14)
Developmental cell 2019 aug
A Comprehensive Structure-Function Study of Neurogenin3 Disease-Causing Alleles during Human Pancreas and Intestinal Organoid Development.
Abstract
Abstract
Neurogenin3 (NEUROG3) is required for endocrine lineage formation of the pancreas and intestine. Patients with NEUROG3 mutations are born with congenital malabsorptive diarrhea due to complete loss of enteroendocrine cells, whereas endocrine pancreas development varies in an allele-specific manner. These findings suggest a context-dependent requirement for NEUROG3 in pancreas versus intestine. We utilized human tissue differentiated from NEUROG3-/- pluripotent stem cells for functional analyses. Most disease-associated alleles had hypomorphic or null phenotype in both tissues, whereas the S171fsX68 mutation had reduced activity in the pancreas but largely null in the intestine. Biochemical studies revealed NEUROG3 variants have distinct molecular defects with altered protein stability, DNA binding, and gene transcription. Moreover, NEUROG3 was highly unstable in the intestinal epithelium, explaining the enhanced sensitivity of intestinal defects relative to the pancreas. These studies emphasize that studies of human mutations in the endogenous tissue context may be required to assess structure-function relationships.
Nanotoxicology 2019
Oral administration of hydroxylated-graphene quantum dots induces intestinal injury accompanying the loss of intestinal stem cells and proliferative progenitor cells.
Abstract
Abstract
Graphene quantum dots (GQDs) have gained significant attention in various biomedical applications. The physicochemical properties of these nanoparticles, including toxic effects, are largely determined by their surface modifications. Previous studies have demonstrated high in vitro cytotoxicity of the hydroxylated GQDs (OH-GQDs). The focus of this study was on the intestinal toxicity of OH-GQDs. Briefly, C57BL/6J mice were given daily oral gavage of 0.05, 0.5 or 5 mg/kg OH-GQD for 7 days, and the indices of intestinal damage were evaluated. Higher doses of the OH-GQDs caused significant intestinal injuries, such as enhanced intestinal permeability, shortened villi and crypt loss. The number of Lgr5+ intestinal stem cells also decreased dramatically upon OH-GQDs exposure, which also inhibited the Ki67+ proliferative progenitor cells. In addition, an increased number of crypt cells harboring the oxidized DNA base 8-OHdG and $\gamma$H2AX foci were also detected in the intestines of OH-GQD-treated mice. Mechanistically, the OH-GQDs up-regulated both total and phosphorylated p53. Consistent with this, the average number of TUNEL+ and cleaved caspase-3+ apoptotic intestinal epithelial cells were significantly increased after OH-GQDs treatment. Finally, a 3-dimensional organoid culture was established using isolated crypts, and OH-GQDs treatment significantly reduced the size of the surviving intestinal organoids. Taken together, the intestinal toxicity of the OH-GQDs should be taken into account during biomedical applications.
Scientific Reports 2018 SEP
IL-27 amplifies cytokine responses to Gram-negative bacterial products and Salmonella typhimurium infection.
Abstract
Abstract
Cytokine responses from monocytes and macrophages exposed to bacteria are of particular importance in innate immunity. Focusing on the impact of the immunoregulatory cytokine interleukin (IL)-27 on control of innate immune system responses, we examined human immune responses to bacterial products and bacterial infection by E. coli and S. typhimurium. Since the effect of IL-27 treatment in human myeloid cells infected with bacteria is understudied, we treated human monocytes and macrophages with IL-27 and either LPS, flagellin, or bacteria, to investigate the effect on inflammatory signaling and cytokine responses. We determined that simultaneous stimulation with IL-27 and LPS derived from E. coli or S. typhimurium resulted in enhanced IL-12p40, TNF-$\alpha$, and IL-6 expression compared to that by LPS alone. To elucidate if IL-27 manipulated the cellular response to infection with bacteria, we infected IL-27 treated human macrophages with S. typhimurium. While IL-27 did not affect susceptibility to S. typhimurium infection or S. typhimurium-induced cell death, IL-27 significantly enhanced proinflammatory cytokine production in infected cells. Taken together, we highlight a role for IL-27 in modulating innate immune responses to bacterial infection.
Nature medicine 2018 OCT
Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma.
Abstract
Abstract
Preventing the immune escape of tumor cells by blocking inhibitory checkpoints, such as the interaction between programmed death ligand-1 (PD-L1) and programmed death-1 (PD-1) receptor, is a powerful anticancer approach. However, many patients do not respond to checkpoint blockade. Tumor PD-L1 expression is a potential efficacy biomarker, but the complex mechanisms underlying its regulation are not completely understood. Here, we show that the eukaryotic translation initiation complex, eIF4F, which binds the 5' cap of mRNAs, regulates the surface expression of interferon-$\gamma$-induced PD-L1 on cancer cells by regulating translation of the mRNA encoding the signal transducer and activator of transcription 1 (STAT1) transcription factor. eIF4F complex formation correlates with response to immunotherapy in human melanoma. Pharmacological inhibition of eIF4A, the RNA helicase component of eIF4F, elicits powerful antitumor immune-mediated effects via PD-L1 downregulation. Thus, eIF4A inhibitors, in development as anticancer drugs, may also act as cancer immunotherapies.
Genes {\&} Immunity 2018 MAY
Mutations in RNA Polymerase III genes and defective DNA sensing in adults with varicella-zoster virus CNS infection
Abstract
Abstract
Recently, deficiency in the cytosolic DNA sensor RNA Polymerase III was described in children with severe primary varicella-zoster virus (VZV) infection in the CNS and lungs. In the present study we examined adult patients with VZV CNS infection caused by viral reactivation. By whole exome sequencing we identified mutations in POL III genes in two of eight patients. These mutations were located in the coding regions of the subunits POLR3A and POLR3E. In functional assays, we found impaired expression of antiviral and inflammatory cytokines in response to the POL III agonist Poly(dA:dT) as well as increased viral replication in patient cells compared to controls. Altogether, this study provides significant extension on the current knowledge on susceptibility to VZV infection by demonstrating mutations in POL III genes associated with impaired immunological sensing of AT-rich DNA in adult patients with VZV CNS infection.
Frontiers in immunology 2018 JUN
Genetically Induced Tumors in the Oncopig Model Invoke an Antitumor Immune Response Dominated by Cytotoxic CD8 T Cells and Differentiated T Cells Alongside a Regulatory Response Mediated by FOXP3+ T Cells and Immunoregulatory Molecules
Abstract
Abstract
In recent years, immunotherapy has shown considerable promise in the management of several malignancies. However, the majority of preclinical studies have been conducted in rodents, the results of which often translate poorly to patients given the substantial differences between murine and human immunology. As the porcine immune system is far more analogous to that of humans, pigs may serve as a supplementary preclinical model for future testing of such therapies. We have generated the genetically modified Oncopig with inducible tumor formation resulting from concomitant KRAS(G12D) and TP53(R167H) mutations under control of an adenoviral vector Cre-recombinase (AdCre). The objective of this study was to characterize the tumor microenvironment in this novel animal model with respect to T-cell responses in particular and to elucidate the potential use of Oncopigs for future preclinical testing of cancer immunotherapies. In this study, we observed pronounced intratumoral T-cell infiltration with a strong CD8$\beta$(+) predominance alongside a representation of highly differentiated $\gamma$$\delta$ T cells. The infiltrating CD8$\beta$(+) T cells displayed increased expression of the cytotoxic marker perforin when compared with the peripheral T-cell pool. Similarly, there was robust granzyme B staining localizing to the tumors; affirming the presence of cytotoxic immune cells within the tumor. In parallel with this antitumor immune response, the tumors displayed enrichment in FOXP3-expressing T cells and increased gene expression of indoleamine 2,3-dioxygenase 1 (IDO1), cytotoxic T-lymphocyte-associated protein 4 (CTLA4), and programmed death-ligand 1 (PDL1). Finally, we investigated the Oncopig immune system in mediating antitumor immunity. We observed pronounced killing of autologous tumor cells, which demonstrates the propensity of the Oncopig immune system to recognize and mount a cytotoxic response against tumor cells. Together, these findings suggest innate and adaptive recognition of the induced tumors with a concomitant in vivo suppression of T-cell effector functions. Combined, the data support that the Oncopig may serve as a valuable model for future preclinical testing of immunotherapies aimed at reactivating tumor-directed cytotoxicity in vivo.