RosetteSep™ Human T Cell Enrichment Cocktail
Immunodensity negative selection cocktail
概要
The RosetteSep™ Human T Cell Enrichment Cocktail is designed to isolate T cells from whole blood by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing non-T cells and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as RosetteSep™ DM-L (Catalog #15705) or Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The purified T cells are present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Isolated cells are untouched
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Isolated cells are untouched
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human T Cell Enrichment Cocktail (Catalog #15021)
- RosetteSep™ Human T Cell Enrichment Cocktail, 2 mL
- RosetteSep™ Human T Cell Enrichment Cocktail (Catalog #15061)
- RosetteSep™ Human T Cell Enrichment Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
T Cells
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human T Cell Enrichment Cocktail | 15021, 15061 | All | English |
| Safety Data Sheet 1 | RosetteSep™ Human T Cell Enrichment Cocktail | 15021 | All | English |
| Safety Data Sheet 2 | RosetteSep™ Human T Cell Enrichment Cocktail | 15021, 15061 | All | English |
数据及文献
Data
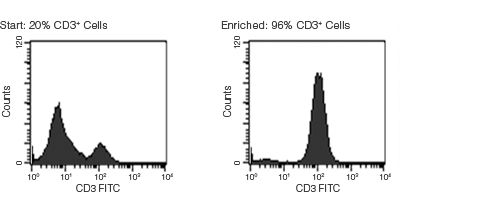
Figure 1. Typical RosetteSep™ T Cell Enrichment Profile
Starting with fresh whole blood the CD3+ cell content of the enriched fraction typically ranges from 90% - 97%. Red blood cells were removed by lysis prior to flow cytometry.
Publications (49)
Cancer research 2020 nov
PET Reporter Gene Imaging and Ganciclovir-Mediated Ablation of Chimeric Antigen Receptor T Cells in Solid Tumors.
Abstract
Abstract
Imaging strategies to monitor chimeric antigen receptor (CAR) T-cell biodistribution and proliferation harbor the potential to facilitate clinical translation for the treatment of both liquid and solid tumors. In addition, the potential adverse effects of CAR T cells highlight the need for mechanisms to modulate CAR T-cell activity. The herpes simplex virus type 1 thymidine kinase (HSV1-tk) gene has previously been translated as a PET reporter gene for imaging of T-cell trafficking in patients with brain tumor. The HSV1-TK enzyme can act as a suicide gene of transduced cells through treatment with the prodrug ganciclovir. Here we report the molecular engineering, imaging, and ganciclovir-mediated destruction of B7H3 CAR T cells incorporating a mutated version of the HSV1-tk gene (sr39tk) with improved enzymatic activity for ganciclovir. The sr39tk gene did not affect B7H3 CAR T-cell functionality and in vitro and in vivo studies in osteosarcoma models showed no significant effect on B7H3 CAR T-cell antitumor activity. PET/CT imaging with 9-(4-[18F]-fluoro-3-[hydroxymethyl]butyl)guanine ([18F]FHBG) of B7H3-sr39tk CAR T cells in an orthotopic model of osteosarcoma revealed tumor homing and systemic immune expansion. Bioluminescence and PET imaging of B7H3-sr39tk CAR T cells confirmed complete tumor ablation with intraperitoneal ganciclovir administration. This imaging and suicide ablation system can provide insight into CAR T-cell migration and proliferation during clinical trials while serving as a suicide switch to limit potential toxicities. SIGNIFICANCE: This study showcases the only genetically engineered system capable of serving the dual role both as an effective PET imaging reporter and as a suicide switch for CAR T cells.
Scientific reports 2020 jun
A Simple and Scalable Strategy for Analysis of Endogenous Protein Dynamics.
Abstract
Abstract
The ability to analyze protein function in a native context is central to understanding cellular physiology. This study explores whether tagging endogenous proteins with a reporter is a scalable strategy for generating cell models that accurately quantitate protein dynamics. Specifically, it investigates whether CRISPR-mediated integration of the HiBiT luminescent peptide tag can easily be accomplished on a large-scale and whether integrated reporter faithfully represents target biology. For this purpose, a large set of proteins representing diverse structures and functions, some of which are known or potential drug targets, were targeted for tagging with HiBiT in multiple cell lines. Successful insertion was detected for 86{\%} of the targets, as determined by luminescence-based plate assays, blotting, and imaging. In order to determine whether endogenously tagged proteins yield more representative models, cells expressing HiBiT protein fusions either from endogenous loci or plasmids were directly compared in functional assays. In the tested cases, only the edited lines were capable of accurately reproducing the anticipated biology. This study provides evidence that cell lines expressing HiBiT fusions from endogenous loci can be rapidly generated for many different proteins and that these cellular models provide insight into protein function that may be unobtainable using overexpression-based approaches.
Scientific reports 2019 nov
PD-1+ melanocortin receptor dependent-Treg cells prevent autoimmune disease.
Abstract
Abstract
Experimental autoimmune uveoretinitis (EAU) is a mouse model of human autoimmune uveitis marked by ocular autoantigen-specific regulatory immunity in the spleen. The melanocortin 5 receptor (MC5r) and adenosine 2 A receptor (A2Ar) are required for induction of post-EAU regulatory T cells (Tregs) which provide resistance to EAU. We show that blocking the PD-1/PD-L1 pathway prevented suppression of EAU by post-EAU Tregs. A2Ar induction of PD-1+FoxP3+ Tregs in uveitis patients was similar compared to healthy controls, but was significantly reduced with melanocortin stimulation. Further, lower body mass index correlated with responsiveness to stimulation of this pathway. These observations indicate an importance of the PD-1/PD-L1 pathway to provide resistance to relapsing uveitis and shows a reduced capacity of uveitis patients to induce Tregs when stimulated through melanocortin receptors, but that it is possible to bypass this part of the pathway through direct stimulation of A2Ar.
Scientific reports 2019 may
Differences in the molecular signatures of mucosal-associated invariant T cells and conventional T cells.
Abstract
Abstract
Mucosal-associated invariant T (MAIT) cells exhibit different characteristics from those of TCRalpha7.2- conventional T cells. They play important roles in various inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease. MAIT cells express a single T cell receptor alpha chain, TCRalpha7.2 segment associated with Jalpha33 and CDR3 with fixed length, which recognizes bacteria-derived vitamin B metabolites. However, the characteristics of MAIT cells and TCRalpha7.2+ CD161- T cells have never been compared. Here, we performed RNA sequencing to compare the properties of MAIT cells, TCRalpha7.2- conventional T cells and TCRalpha7.2+ CD161- T cells. Genome-wide transcriptomes of MAIT cells, TCRalpha7.2- conventional T cells, and TCRalpha7.2+ CD161- T cells were compared and analyzed using causal network analysis. This is the first report comparing the transcriptomes of MAIT cells, TCRalpha7.2- conventional T cells and TCRalpha7.2+ CD161- T cells. We also identified the predominant signaling pathways of MAIT cells, which differed from those of TCRalpha7.2- conventional T cells and TCRalpha7.2+ CD161- T cells, through a gene set enrichment test and upstream regulator analysis and identified the genes responsible for the characteristic MAIT cell phenotypes. Our study advances the complete understanding of MAIT biology.
Nature biotechnology 2019 feb
Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing.
Abstract
Abstract
Broad use of CRISPR-Cas12a (formerly Cpf1) nucleases1 has been hindered by the requirement for an extended TTTV protospacer adjacent motif (PAM)2. To address this limitation, we engineered an enhanced Acidaminococcus sp. Cas12a variant (enAsCas12a) that has a substantially expanded targeting range, enabling targeting of many previously inaccessible PAMs. On average, enAsCas12a exhibits a twofold higher genome editing activity on sites with canonical TTTV PAMs compared to wild-type AsCas12a, and we successfully grafted a subset of mutations from enAsCas12a onto other previously described AsCas12a variants3 to enhance their activities. enAsCas12a improves the efficiency of multiplex gene editing, endogenous gene activation and C-to-T base editing, and we engineered a high-fidelity version of enAsCas12a (enAsCas12a-HF1) to reduce off-target effects. Both enAsCas12a and enAsCas12a-HF1 function in HEK293T and primary human T cells when delivered as ribonucleoprotein (RNP) complexes. Collectively, enAsCas12a provides an optimized version of Cas12a that should enable wider application of Cas12a enzymes for gene and epigenetic editing.
Nature medicine 2018 OCT
Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma.
Abstract
Abstract
Preventing the immune escape of tumor cells by blocking inhibitory checkpoints, such as the interaction between programmed death ligand-1 (PD-L1) and programmed death-1 (PD-1) receptor, is a powerful anticancer approach. However, many patients do not respond to checkpoint blockade. Tumor PD-L1 expression is a potential efficacy biomarker, but the complex mechanisms underlying its regulation are not completely understood. Here, we show that the eukaryotic translation initiation complex, eIF4F, which binds the 5' cap of mRNAs, regulates the surface expression of interferon-$\gamma$-induced PD-L1 on cancer cells by regulating translation of the mRNA encoding the signal transducer and activator of transcription 1 (STAT1) transcription factor. eIF4F complex formation correlates with response to immunotherapy in human melanoma. Pharmacological inhibition of eIF4A, the RNA helicase component of eIF4F, elicits powerful antitumor immune-mediated effects via PD-L1 downregulation. Thus, eIF4A inhibitors, in development as anticancer drugs, may also act as cancer immunotherapies.