RosetteSep™ Human Monocyte Depletion Cocktail
Immunodensity depletion cocktail
概要
The RosetteSep™ Human Monocyte Depletion Cocktail is designed to deplete monocytes from whole blood. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD36 and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The monocyte depleted fraction is present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human Monocyte Depletion Cocktail (Catalog #15628)
- RosetteSep™ Human Monocyte Depletion Cocktail, 2 mL
- RosetteSep™ Human Monocyte Depletion Cocktail (Catalog #15668)
- RosetteSep™ Human Monocyte Depletion Cocktail, 5 x 2 mL
Magnet Compatibility
Subtype
Cell Isolation Kits
Cell Type
Monocytes
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Depletion
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human Monocyte Depletion Cocktail | 15628, 15668 | All | English |
| Safety Data Sheet | RosetteSep™ Human Monocyte Depletion Cocktail | 15628 | All | English |
数据及文献
Data
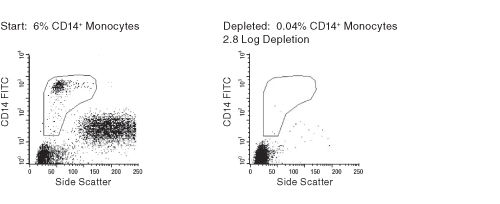
Figure 1. FACS Profile Results Using RosetteSep™ Human CD36+ Cells Depletion Cocktail
Publications (3)
Journal of medicinal chemistry 2019 dec
Enzymatic Preparation of 2'-5',3'-5'-Cyclic Dinucleotides, Their Binding Properties to Stimulator of Interferon Genes Adaptor Protein, and Structure/Activity Correlations.
Abstract
Abstract
Cyclic dinucleotides are second messengers in the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, which plays an important role in recognizing tumor cells and viral or bacterial infections. They bind to the STING adaptor protein and trigger expression of cytokines via TANK binding kinase 1 (TBK1)/interferon regulatory factor 3 (IRF3) and inhibitor of nuclear factor-$\kappa$B (I$\kappa$B) kinase (IKK)/nuclear factor-$\kappa$B (NF$\kappa$B) signaling cascades. In this work, we describe an enzymatic preparation of 2'-5',3'-5'-cyclic dinucleotides (2'3'CDNs) with use of cyclic GMP-AMP synthases (cGAS) from human, mouse, and chicken. We profile substrate specificity of these enzymes by employing a small library of nucleotide-5'-triphosphate (NTP) analogues and use them to prepare 33 2'3'CDNs. We also determine affinity of these CDNs to five different STING haplotypes in cell-based and biochemical assays and describe properties needed for their optimal activity toward all STING haplotypes. Next, we study their effect on cytokine and chemokine induction by human peripheral blood mononuclear cells (PBMCs) and evaluate their cytotoxic effect on monocytes. Additionally, we report X-ray crystal structures of two new CDNs bound to STING protein and discuss structure-activity relationship by using quantum and molecular mechanical (QM/MM) computational modeling.
Scientific reports 2017 FEB
Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice.
Abstract
Abstract
Targeting of myeloid-dendritic cell receptor DC-SIGN by numerous chronic infectious agents, including Porphyromonas gingivalis, is shown to drive-differentiation of monocytes into dysfunctional mDCs. These mDCs exhibit alterations of their fine-tuned homeostatic function and contribute to dysregulated immune-responses. Here, we utilize P. gingivalis mutant strains to show that pathogen-differentiated mDCs from primary human-monocytes display anti-apoptotic profile, exhibited by elevated phosphorylated-Foxo1, phosphorylated-Akt1, and decreased Bim-expression. This results in an overall inhibition of DC-apoptosis. Direct stimulation of complex component CD40 on DCs leads to activation of Akt1, suggesting CD40 involvement in anti-apoptotic effects observed. Further, these DCs drove dampened CD8(+) T-cell and Th1/Th17 effector-responses while inducing CD25(+)Foxp3(+)CD127(-) Tregs. In vitro Treg induction was mediated by DC expression of indoleamine 2,3-dioxygenase, and was confirmed in IDO-KO mouse model. Pathogen-infected &CMFDA-labeled MoDCs long-lasting survival was confirmed in a huMoDC reconstituted humanized mice. In conclusion, our data implicate PDDCs as an important target for resolution of chronic infection.
Proceedings of the National Academy of Sciences of the United States of America 2009 JUN
Impaired interferon signaling is a common immune defect in human cancer.
Abstract
Abstract
Immune dysfunction develops in patients with many cancer types and may contribute to tumor progression and failure of immunotherapy. Mechanisms underlying cancer-associated immune dysfunction are not fully understood. Efficient IFN signaling is critical to lymphocyte function; animals rendered deficient in IFN signaling develop cancer at higher rates. We hypothesized that altered IFN signaling may be a key mechanism of immune dysfunction common to cancer. To address this, we assessed the functional responses to IFN in peripheral blood lymphocytes from patients with 3 major cancers: breast cancer, melanoma, and gastrointestinal cancer. Type-I IFN (IFN-alpha)-induced signaling was reduced in T cells and B cells from all 3 cancer-patient groups compared to healthy controls. Type-II IFN (IFN-gamma)-induced signaling was reduced in B cells from all 3 cancer patient groups, but not in T cells or natural killer cells. Impaired-IFN signaling was equally evident in stage II, III, and IV breast cancer patients, and downstream functional defects in T cell activation were identified. Taken together, these findings indicate that defects in lymphocyte IFN signaling arise in patients with breast cancer, melanoma, and gastrointestinal cancer, and these defects may represent a common cancer-associated mechanism of immune dysfunction.




