RosetteSep™ Human Granulocyte Depletion Cocktail
Immunodensity depletion cocktail
概要
The RosetteSep™ Human Granulocyte Depletion Cocktail is designed to deplete granulocytes from whole blood. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD66b and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The granulocyte-depleted fraction is present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human Granulocyte Depletion Cocktail (Catalog #15624)
- RosetteSep™ Human Granulocyte Depletion Cocktail, 2 mL
- RosetteSep™ Human Granulocyte Depletion Cocktail (Catalog #15664)
- RosetteSep™ Human Granulocyte Depletion Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
Granulocytes and Subsets
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Depletion
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human Granulocyte Depletion Cocktail | 15624, 15664 | All | English |
| Safety Data Sheet | RosetteSep™ Human Granulocyte Depletion Cocktail | 15624 | All | English |
| Safety Data Sheet | RosetteSep™ Human Granulocyte Depletion Cocktail | 15664 | All | English |
数据及文献
Data
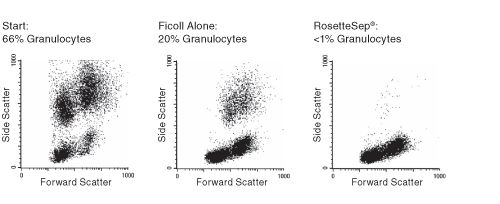
Figure 1. FACS Profile Results Using RosetteSep™ Human Granulocyte Depletion Cocktail
Publications (3)
PloS one 2016
Interleukins 7 and 15 Maintain Human T Cell Proliferative Capacity through STAT5 Signaling.
Abstract
Abstract
T lymphocytes require signals from self-peptides and cytokines, most notably interleukins 7 and 15 (IL-7, IL-15), for survival. While mouse T cells die rapidly if IL-7 or IL-15 is withdrawn, human T cells can survive prolonged withdrawal of IL-7 and IL-15. Here we show that IL-7 and IL-15 are required to maintain human T cell proliferative capacity through the STAT5 signaling pathway. T cells from humanized mice proliferate better if stimulated in the presence of human IL-7 or IL-15 or if T cells are exposed to human IL-7 or IL-15 in mice. Freshly isolated T cells from human peripheral blood lose proliferative capacity if cultured for 24 hours in the absence of IL-7 or IL-15. We further show that phosphorylation of STAT5 correlates with proliferation and inhibition of STAT5 reduces proliferation. These results reveal a novel role of IL-7 and IL-15 in maintaining human T cell function, provide an explanation for T cell dysfunction in humanized mice, and have significant implications for in vitro studies with human T cells.
Proceedings of the National Academy of Sciences of the United States of America 2009 JUN
Impaired interferon signaling is a common immune defect in human cancer.
Abstract
Abstract
Immune dysfunction develops in patients with many cancer types and may contribute to tumor progression and failure of immunotherapy. Mechanisms underlying cancer-associated immune dysfunction are not fully understood. Efficient IFN signaling is critical to lymphocyte function; animals rendered deficient in IFN signaling develop cancer at higher rates. We hypothesized that altered IFN signaling may be a key mechanism of immune dysfunction common to cancer. To address this, we assessed the functional responses to IFN in peripheral blood lymphocytes from patients with 3 major cancers: breast cancer, melanoma, and gastrointestinal cancer. Type-I IFN (IFN-alpha)-induced signaling was reduced in T cells and B cells from all 3 cancer-patient groups compared to healthy controls. Type-II IFN (IFN-gamma)-induced signaling was reduced in B cells from all 3 cancer patient groups, but not in T cells or natural killer cells. Impaired-IFN signaling was equally evident in stage II, III, and IV breast cancer patients, and downstream functional defects in T cell activation were identified. Taken together, these findings indicate that defects in lymphocyte IFN signaling arise in patients with breast cancer, melanoma, and gastrointestinal cancer, and these defects may represent a common cancer-associated mechanism of immune dysfunction.
Proceedings of the National Academy of Sciences of the United States of America 2006 OCT
Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways.
Abstract
Abstract
Monitoring genome-wide, cell-specific responses to human disease, although challenging, holds great promise for the future of medicine. Patients with injuries severe enough to develop multiple organ dysfunction syndrome have multiple immune derangements, including T cell apoptosis and anergy combined with depressed monocyte antigen presentation. Genome-wide expression analysis of highly enriched circulating leukocyte subpopulations, combined with cell-specific pathway analyses, offers an opportunity to discover leukocyte regulatory networks in critically injured patients. Severe injury induced significant changes in T cell (5,693 genes), monocyte (2,801 genes), and total leukocyte (3,437 genes) transcriptomes, with only 911 of these genes common to all three cell populations (12%). T cell-specific pathway analyses identified increased gene expression of several inhibitory receptors (PD-1, CD152, NRP-1, and Lag3) and concomitant decreases in stimulatory receptors (CD28, CD4, and IL-2Ralpha). Functional analysis of T cells and monocytes confirmed reduced T cell proliferation and increased cell surface expression of negative signaling receptors paired with decreased monocyte costimulation ligands. Thus, genome-wide expression from highly enriched cell populations combined with knowledge-based pathway analyses leads to the identification of regulatory networks differentially expressed in injured patients. Importantly, application of cell separation, genome-wide expression, and cell-specific pathway analyses can be used to discover pathway alterations in human disease.




