RosetteSep™ Human CD45 Depletion Cocktail
Immunodensity depletion cocktail
概要
The RosetteSep™ Human CD45 Depletion Cocktail is designed to enrich epithelial circulating tumor cells (CTCs) from whole blood by depleting CD45+ cells. Unwanted cells are targeted for depletion with Tetrameric Antibody Complexes recognizing CD45, CD66b and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The purified epithelial tumor cells are present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human CD45 Depletion Cocktail (Catalog #15122)
- RosetteSep™ Human CD45 Depletion Cocktail, 2 mL
- RosetteSep™ Human CD45 Depletion Cocktail (Catalog #15162)
- RosetteSep™ Human CD45 Depletion Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
Cancer Cells and Cell Lines
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Depletion
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Cancer Research, Immunology, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human CD45 Depletion Cocktail | 15122, 15162 | All | English |
| Safety Data Sheet | RosetteSep™ Human CD45 Depletion Cocktail | 15122 | All | English |
数据及文献
Data
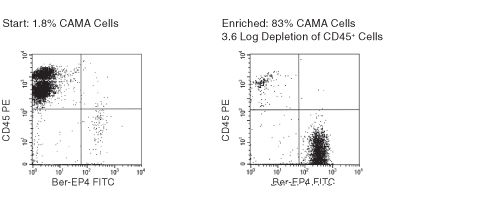
Figure 1. FACS Profile Results with RosetteSep™ Human CD45+ Cell Depletion Cocktail
Ber-EP4 is an antibody against an epithelial cell surface antigen.
Publications (12)
Journal of clinical medicine 2020 jan
Sequential Circulating Tumor Cell Counts in Patients with Locally Advanced or Metastatic Hepatocellular Carcinoma: Monitoring the Treatment Response.
Abstract
Abstract
Hepatocellular carcinoma (HCC) is among the most common causes of cancer death in men. Whether or not a longitudinal follow-up of circulating tumor cells (CTCs) before and at different time points during systemic/targeted therapy is useful for monitoring the treatment response of patients with locally advanced or metastatic HCC has been evaluated in this study. Blood samples (n = 104) were obtained from patients with locally advanced or metastatic HCC (n = 30) for the enrichment of CTCs by a negative selection method. Analysis of the blood samples from patients with defined disease status (n = 81) revealed that those with progressive disease (PD, n = 37) had significantly higher CTC counts compared to those with a partial response (PR) or stable disease (SD; n = 44 for PR + SD, p = 0.0002). The median CTC count for patients with PD and for patients with PR and SD was 50 (interquartile range 21-139) and 15 (interquartile range 4-41) cells/mL of blood, respectively. A longitudinal analysis of patients (n = 17) after a series of blood collections demonstrated that a change in the CTC count correlated with the patient treatment response in most of the cases and was particularly useful for monitoring patients without elevated serum alpha-fetoprotein (AFP) levels. Sequential CTC enumeration during treatment can supplement standard medical tests and benefit the management of patients with locally advanced or metastatic HCC, in particular for the AFP-low cases.
JCI insight 2019 jun
Sensitive and easy screening for circulating tumor cells by flow cytometry.
Abstract
Abstract
Circulating Tumor Cells (CTCs) represent an easy, repeatable and representative access to information regarding solid tumors. However, their detection remains difficult because of their paucity, their short half-life, and the lack of reliable surface biomarkers. Flow cytometry (FC) is a fast, sensitive and affordable technique, ideal for rare cells detection. Adapted to CTCs detection (i.e. extremely rare cells), most FC-based techniques require a time-consuming pre-enrichment step, followed by a 2-hours staining procedure, impeding on the efficiency of CTCs detection. We overcame these caveats and reduced the procedure to less than one hour, with minimal manipulation. First, cells were simultaneously fixed, permeabilized, then stained. Second, using low-speed FC acquisition conditions and two discriminators (cell size and pan-cytokeratin expression), we suppressed the pre-enrichment step. Applied to blood from donors with or without known malignant diseases, this protocol ensures a high recovery of the cells of interest independently of their epithelial-mesenchymal plasticity and can predict which samples are derived from cancer donors. This proof-of-concept study lays the bases of a sensitive tool to detect CTCs from a small amount of blood upstream of in-depth analyses.
Cells 2019 jan
Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells.
Abstract
Abstract
Various preclinical models have been developed to clarify the pathophysiology of prostate cancer (PCa). Traditional PCa cell lines from clinical metastatic lesions, as exemplified by DU-145, PC-3, and LNCaP cells, are useful tools to define mechanisms underlying tumorigenesis and drug resistance. Cell line-based experiments, however, have limitations for preclinical studies because those cells are basically adapted to 2-dimensional monolayer culture conditions, in which the majority of primary PCa cells cannot survive. Recent tissue engineering enables generation of PCa patient-derived xenografts (PDXs) from both primary and metastatic lesions. Compared with fresh PCa tissue transplantation in athymic mice, co-injection of PCa tissues with extracellular matrix in highly immunodeficient mice has remarkably improved the success rate of PDX generation. PDX models have advantages to appropriately recapitulate the molecular diversity, cellular heterogeneity, and histology of original patient tumors. In contrast to PDX models, patient-derived organoid and spheroid PCa models in 3-dimensional culture are more feasible tools for in vitro studies for retaining the characteristics of patient tumors. In this article, we review PCa preclinical model cell lines and their sublines, PDXs, and patient-derived organoid and spheroid models. These PCa models will be applied to the development of new strategies for cancer precision medicine.
Scientific reports 2019 dec
The Prognostic Value of Circulating Tumor Cells in Asian Neuroendocrine Tumors.
Abstract
Abstract
Circulating tumor cells (CTC) play important roles in various cancers; however, few studies have assessed their clinical utility in neuroendocrine tumors. This study aimed to prospectively evaluate the prognostic value of CTC counts in Asian patients with neuroendocrine tumors before and during anti-cancer therapy. Patients who were diagnosed with unresectable histological neuroendocrine tumors between September 2011 and September 2017 were enrolled. CTC testing was performed before and during anti-cancer therapy using a negative selection protocol. Chromogranin A levels were also assessed. Univariate and multivariate Cox's proportional hazard model with forward LR model was performed to investigate the impact of independent factors on overall survival and progression-free survival. Kaplan-Meier method with log-rank tests were used to determine the difference among different clinicopathological signatures and CTC cutoff. The baseline CTC detection rate was 94.3{\%} (33/35). CTC counts were associated with cancer stages (I-III vs. IV, P = 0.015), liver metastasis (P = 0.026), and neuroendocrine tumor grading (P = 0.03). The median progression-free survival and overall survivals were 12.3 and 30.4 months, respectively. In multivariate Cox regression model, neuroendocrine tumors grading and baseline CTC counts were both independent prognostic factors for progression-free survival (PFS, P = 0.005 and 0.015, respectively) and overall survival (OS, P = 0.018 and 0.023, respectively). In Kaplan-Meier analysis, lower baseline chromogranin A levels were associated with longer PFS (P = 0.024). Baseline CTC counts are associated with the clinicopathologic features of neuroendocrine tumors and are an independent prognostic factor for this malignancy.
Seminars in cancer biology 2017 APR
How to study and overcome tumor heterogeneity with circulating biomarkers: The breast cancer case.
Abstract
Abstract
Breast cancer ranks first among female cancer-related deaths in Western countries. As the primary tumor can often be controlled by surgical resection, the survival of women with breast cancer is closely linked to the incidence of distant metastases. Molecular screening by next generation sequencing highlighted the spatial and temporal heterogeneity of solid tumors as well as the clonal evolution of cancer cells during progression and under treatment pressure. Such findings question whether an optimal assessment of disease progression and a screening for druggable mutations should be based on molecular features of primary or recurrent/metastatic lesions and therefore represent a crucial element for failure or success of personalized medicine. In fact, new targeted therapies may induce only short-term benefit annulled by the emergence of resistant clones with new driver mutations which would need to be rapidly and reliably identified. Serial tissue sampling is therefore essential but, unfortunately, also represents a problem since biopsies from solid lesions, which are invasive and potentially painful and risky, cannot be easily repeatedly sampled, are inaccessible or may not fully reflect tumor heterogeneity. The need to early detect and strike this moving target" is now directing the scientific community towards liquid biopsy-based biomarkers which include circulating tumor cells (CTC) and cell-free circulating tumor DNA (ctDNA) can be repeatedly assessed through non-invasive and easy-to-perform procedures and may act as reliable read-outs of functional and molecular features of recurrent/metastatic lesions. In this review we summarize the outcome of CTCs and ctDNA in breast cancer with special reference on their role on unveiling and overcoming tumor heterogeneity on their potential relevance for tumor surveillance and monitoring and for the selection of therapeutic options. Finally we propose integration between blood-based molecular and clinical approaches for monitoring disease progression according to the specific pattern of recurrence of the most aggressive breast cancer molecular subtypes."
Nature protocols 2016 FEB
Organoid culture systems for prostate epithelial and cancer tissue.
Abstract
Abstract
This protocol describes a strategy for the generation of 3D prostate organoid cultures from healthy mouse and human prostate cells (either bulk or FACS-sorted single luminal and basal cells), metastatic prostate cancer lesions and circulating tumor cells. Organoids derived from healthy material contain the differentiated luminal and basal cell types, whereas organoids derived from prostate cancer tissue mimic the histology of the tumor. We explain how to establish these cultures in the fully defined serum-free conditioned medium that is required to sustain organoid growth. Starting with the plating of digested tissue material, full-grown organoids can usually be obtained in ∼2 weeks. The culture protocol we describe here is currently the only one that allows the growth of both the luminal and basal prostatic epithelial lineages, as well as the growth of advanced prostate cancers. Organoids established using this protocol can be used to study many different aspects of prostate biology, including homeostasis, tumorigenesis and drug discovery.




