RosetteSep™ Human CD4 Depletion Cocktail
Immunodensity depletion cocktail
概要
The RosetteSep™ Human CD4 Depletion Cocktail is designed to deplete CD4+ cells from whole blood. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD4 and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The CD4+ depleted fraction is present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human CD4 Depletion Cocktail (Catalog #15622)
- RosetteSep™ Human CD4 Depletion Cocktail, 2 mL
- RosetteSep™ Human CD4 Depletion Cocktail (Catalog #15662)
- RosetteSep™ Human CD4 Depletion Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD4+
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Depletion
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human CD4 Depletion Cocktail | 15622, 15662 | All | English |
| Safety Data Sheet | RosetteSep™ Human CD4 Depletion Cocktail | 15622 | All | English |
数据及文献
Data
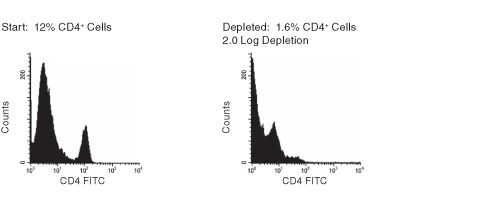
Figure 1. FACS Histogram Results Using RosetteSep™ Human CD4+ Cell Depletion Cocktail
Publications (2)
Journal of immunology (Baltimore, Md. : 1950) 2019 jul
Metformin Inhibits the Type 1 IFN Response in Human CD4+ T Cells.
Abstract
Abstract
In systemic lupus erythematosus, defective clearance of apoptotic debris and activation of innate cells result in a chronically activated type 1 IFN response, which can be measured in PBMCs of most patients. Metformin, a widely used prescription drug for Type 2 diabetes, has a therapeutic effect in several mouse models of lupus through mechanisms involving inhibition of oxidative phosphorylation and a decrease in CD4+ T cell activation. In this study, we report that in CD4+ T cells from human healthy controls and human systemic lupus erythematosus patients, metformin inhibits the transcription of IFN-stimulated genes (ISGs) after IFN-alpha treatment. Accordingly, metformin inhibited the phosphorylation of pSTAT1 (Y701) and its binding to IFN-stimulated response elements that control ISG expression. These effects were independent of AMPK activation or mTORC1 inhibition but were replicated using inhibitors of the electron transport chain respiratory complexes I, III, and IV. This indicates that mitochondrial respiration is required for ISG expression in CD4+ T cells and provides a novel mechanism by which metformin may exert a therapeutic effect in autoimmune diseases.
Mechanisms of development 2019
Developmental regulation of Wnt signaling by Nagk and the UDP-GlcNAc salvage pathway.
Abstract
Abstract
In a screen for human kinases that regulate Xenopus laevis embryogenesis, we identified Nagk and other components of the UDP-GlcNAc glycosylation salvage pathway as regulators of anteroposterior patterning and Wnt signaling. We find that the salvage pathway does not affect other major embryonic signaling pathways (Fgf, TGF$\beta$, Notch, or Shh), thereby demonstrating specificity for Wnt signaling. We show that the role of the salvage pathway in Wnt signaling is evolutionarily conserved in zebrafish and Drosophila. Finally, we show that GlcNAc is essential for the growth of intestinal enteroids, which are highly dependent on Wnt signaling for growth and maintenance. We propose that the Wnt pathway is sensitive to alterations in the glycosylation state of a cell and acts as a nutritional sensor in order to couple growth/proliferation with its metabolic status. We also propose that the clinical manifestations observed in congenital disorders of glycosylation (CDG) in humans may be due, in part, to their effects on Wnt signaling during development.




