RosetteSep™ Human CD3 Depletion Cocktail
Immunodensity depletion cocktail
概要
The RosetteSep™ Human CD3 Depletion Cocktail is designed to deplete CD3+ cells from whole blood. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD3 and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as RosetteSep™ DM-L (Catalog #15705) or Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The CD3-depleted fraction is present as a highly enriched population at the interface between the plasma and the buoyant density medium.
Advantages
• Fast and easy-to-use
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Requires no special equipment or training
• Untouched, viable cells
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ Human CD3 Depletion Cocktail (Catalog #15621)
- RosetteSep™ Human CD3 Depletion Cocktail, 2 mL
- RosetteSep™ Human CD3 Depletion Cocktail (Catalog #15661)
- RosetteSep™ Human CD3 Depletion Cocktail, 5 x 2 mL
Subtype
Cell Isolation Kits
Cell Type
T Cells
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Depletion
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human CD3 Depletion Cocktail | 15621, 15661 | All | English |
| Safety Data Sheet | RosetteSep™ Human CD3 Depletion Cocktail | 15621 | All | English |
数据及文献
Data
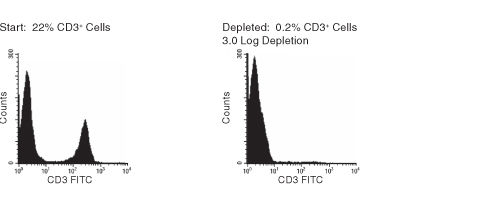
Figure 1. FACS Histogram Results Using RosetteSep™ Human CD3+ Cell Depletion Cocktail
Publications (8)
Nature microbiology 2019
IFN-I and IL-22 mediate protective effects of intestinal viral infection.
Abstract
Abstract
Products derived from bacterial members of the gut microbiota evoke immune signalling pathways of the host that promote immunity and barrier function in the intestine. How immune reactions to enteric viruses support intestinal homeostasis is unknown. We recently demonstrated that infection by murine norovirus (MNV) reverses intestinal abnormalities following depletion of bacteria, indicating that an intestinal animal virus can provide cues to the host that are typically attributed to the microbiota. Here, we elucidate mechanisms by which MNV evokes protective responses from the host. We identify an important role for the viral protein NS1/2 in establishing local replication and a type I interferon (IFN-I) response in the colon. We further show that IFN-I acts on intestinal epithelial cells to increase the proportion of CCR2-dependent macrophages and interleukin (IL)-22-producing innate lymphoid cells, which in turn promote pSTAT3 signalling in intestinal epithelial cells and protection from intestinal injury. In addition, we demonstrate that MNV provides a striking IL-22-dependent protection against early-life lethal infection by Citrobacter rodentium. These findings demonstrate novel ways in which a viral member of the microbiota fortifies the intestinal barrier during chemical injury and infectious challenges.
Scientific reports 2018 OCT
Comparative transcriptomic profile of tolerogenic dendritic cells differentiated with vitamin D3, dexamethasone and rapamycin.
Abstract
Abstract
Tolerogenic dendritic cell (tolDC)-based therapies have become a promising approach for the treatment of autoimmune diseases by their potential ability to restore immune tolerance in an antigen-specific manner. However, the broad variety of protocols used to generate tolDC in vitro and their functional and phenotypical heterogeneity are evidencing the need to find robust biomarkers as a key point towards their translation into the clinic, as well as better understanding the mechanisms involved in the induction of immune tolerance. With that aim, in this study we have compared the transcriptomic profile of tolDC induced with either vitamin D3 (vitD3-tolDC), dexamethasone (dexa-tolDC) or rapamycin (rapa-tolDC) through a microarray analysis in 5 healthy donors. The results evidenced that common differentially expressed genes could not be found for the three different tolDC protocols. However, individually, CYP24A1, MUCL1 and MAP7 for vitD3-tolDC; CD163, CCL18, C1QB and C1QC for dexa-tolDC; and CNGA1 and CYP7B1 for rapa-tolDC, constituted good candidate biomarkers for each respective cellular product. In addition, a further gene set enrichment analysis of the data revealed that dexa-tolDC and vitD3-tolDC share several immune regulatory and anti-inflammatory pathways, while rapa-tolDC seem to be playing a totally different role towards tolerance induction through a strong immunosuppression of their cellular processes.
Frontiers in immunology 2018
Ethyl Pyruvate Stimulates Regulatory T Cells and Ameliorates Type 1 Diabetes Development in Mice.
Abstract
Abstract
Type 1 diabetes (T1D) is an autoimmune disease in which a strong inflammatory response causes the death of insulin-producing pancreatic beta-cells, while inefficient regulatory mechanisms allow that response to become chronic. Ethyl pyruvate (EP), a stable pyruvate derivate and certified inhibitor of an alarmin-high mobility group box 1 (HMGB1), exerts anti-oxidant and anti-inflammatory properties in animal models of rheumatoid arthritis and encephalomyelitis. To test its therapeutic potential in T1D, EP was administered intraperitoneally to C57BL/6 mice with multiple low-dose streptozotocin (MLDS)-induced T1D. EP treatment decreased T1D incidence, reduced the infiltration of cells into the pancreatic islets and preserved beta-cell function. Apart from reducing HMGB1 expression, EP treatment successfully interfered with the inflammatory response within the local pancreatic lymph nodes and in the pancreas. Its effect was restricted to boosting the regulatory arm of the immune response through up-regulation of tolerogenic dendritic cells (CD11c+CD11b-CD103+) within the pancreatic infiltrates and through the enhancement of regulatory T cell (Treg) levels (CD4+CD25highFoxP3+). These EP-stimulated Treg displayed enhanced suppressive capacity reflected in increased levels of CTLA-4, secreted TGF-beta, and IL-10 and in the more efficient inhibition of effector T cell proliferation compared to Treg from diabetic animals. Higher levels of Treg were a result of increased differentiation and proliferation (Ki67+ cells), but also of the heightened potency for migration due to increased expression of adhesion molecules (CD11a and CD62L) and CXCR3 chemokine receptor. Treg isolated from EP-treated mice had the activated phenotype and T-bet expression more frequently, suggesting that they readily suppressed IFN-gamma-producing cells. The effect of EP on Treg was also reproduced in vitro. Overall, our results show that EP treatment reduced T1D incidence in C57BL/6 mice predominantly by enhancing Treg differentiation, proliferation, their suppressive capacity, and recruitment into the pancreas.
Leukemia 2015 OCT
Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab.
Abstract
Abstract
Daratumumab is an anti-CD38 monoclonal antibody with lytic activity against multiple myeloma (MM) cells, including ADCC (antibody-dependent cellular cytotoxicity) and CDC (complement-dependent cytotoxicity). Owing to a marked heterogeneity of response to daratumumab therapy in MM, we investigated determinants of the sensitivity of MM cells toward daratumumab-mediated ADCC and CDC. In bone marrow samples from 144 MM patients, we observed no difference in daratumumab-mediated lysis between newly diagnosed or relapsed/refractory patients. However, we discovered, next to an expected effect of effector (natural killer cells/monocytes) to target (MM cells) ratio on ADCC, a significant association between CD38 expression and daratumumab-mediated ADCC (127 patients), as well as CDC (56 patients). Similarly, experiments with isogenic MM cell lines expressing different levels of CD38 revealed that the level of CD38 expression is an important determinant of daratumumab-mediated ADCC and CDC. Importantly, all-trans retinoic acid (ATRA) increased CD38 expression levels but also reduced expression of the complement-inhibitory proteins CD55 and CD59 in both cell lines and primary MM samples. This resulted in a significant enhancement of the activity of daratumumab in vitro and in a humanized MM mouse model as well. Our results provide the preclinical rationale for further evaluation of daratumumab combined with ATRA in MM patients.
PloS one 2010 JAN
Human antigen-specific regulatory T cells generated by T cell receptor gene transfer.
Abstract
Abstract
BACKGROUND: Therapies directed at augmenting regulatory T cell (Treg) activities in vivo as a systemic treatment for autoimmune disorders and transplantation may be associated with significant off-target effects, including a generalized immunosuppression that may compromise beneficial immune responses to infections and cancer cells. Adoptive cellular therapies using purified expanded Tregs represents an attractive alternative to systemic treatments, with results from animal studies noting increased therapeutic potency of antigen-specific Tregs over polyclonal populations. However, current methodologies are limited in terms of the capacity to isolate and expand a sufficient quantity of endogenous antigen-specific Tregs for therapeutic intervention. Moreover, FOXP3+ Tregs fall largely within the CD4+ T cell subset and are thus routinely MHC class II-specific, whereas class I-specific Tregs may function optimally in vivo by facilitating direct tissue recognition. METHODOLOGY/PRINCIPAL FINDINGS: To overcome these limitations, we have developed a novel means for generating large numbers of antigen-specific Tregs involving lentiviral T cell receptor (TCR) gene transfer into in vitro expanded polyclonal natural Treg populations. Tregs redirected with a high-avidity class I-specific TCR were capable of recognizing the melanoma antigen tyrosinase in the context of HLA-A*0201 and could be further enriched during the expansion process by antigen-specific reactivation with peptide loaded artificial antigen presenting cells. These in vitro expanded Tregs continued to express FOXP3 and functional TCRs, and maintained the capacity to suppress conventional T cell responses directed against tyrosinase, as well as bystander T cell responses. Using this methodology in a model tumor system, murine Tregs designed to express the tyrosinase TCR effectively blocked antigen-specific effector T cell (Teff) activity as determined by tumor cell growth and luciferase reporter-based imaging. CONCLUSIONS/SIGNIFICANCE: These results support the feasibility of class I-restricted TCR transfer as a promising strategy to redirect the functional properties of Tregs and provide for a more efficacious adoptive cell therapy.
Molecular and cellular biology 2006 MAR
Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase C theta-mediated phosphorylation.
Abstract
Abstract
Protein kinase C theta (PKC theta) is unique among PKC isozymes in its translocation to the center of the immune synapse in T cells and its unique downstream signaling. Here we show that the hematopoietic protein tyrosine phosphatase (HePTP) also accumulates in the immune synapse in a PKC theta-dependent manner upon antigen recognition by T cells and is phosphorylated by PKC theta at Ser-225, which is required for lipid raft translocation. Immune synapse translocation was completely absent in antigen-specific T cells from PKC theta-/- mice. In intact T cells, HePTP-S225A enhanced T-cell receptor (TCR)-induced NFAT/AP-1 transactivation, while the acidic substitution mutant was as efficient as wild-type HePTP. We conclude that HePTP is phosphorylated in the immune synapse by PKC theta and thereby targeted to lipid rafts to temper TCR signaling. This represents a novel mechanism for the active immune synapse recruitment and activation of a phosphatase in TCR signaling.




