RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36
Immunodensity negative selection cocktail
概要
The RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36 is designed to enrich circulating epithelial tumor cells from fresh whole blood by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD2, CD16, CD19, CD36, CD38, CD45, CD66b and glycophorin A on red blood cells (RBCs). When centrifuged over a density gradient medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The purified tumor cells are present as a highly enriched population at the interface between the plasma and the density gradient medium.
This cocktail is compatible with small-cell carcinoma samples, including lung cancer samples. If you are working with breast cancer samples, use the RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD56 (Catalog #15137).
This cocktail is compatible with small-cell carcinoma samples, including lung cancer samples. If you are working with breast cancer samples, use the RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD56 (Catalog #15137).
Advantages
• Fast and easy-to-use
• Up to 98% purity
• No columns required
• Can be combined with SepMate™ for consistent, high-throughput sample processing
• Up to 98% purity
• No columns required
• Can be combined with SepMate™ for consistent, high-throughput sample processing
Components
- RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36 (Catalog #15127)
- RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36, 2 mL
- RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36 (Catalog #15167)
- RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36, 5 x 2 mL
Magnet Compatibility
Subtype
Cell Isolation Kits
Cell Type
Cancer Cells and Cell Lines
Species
Human
Sample Source
Buffy Coat, Whole Blood
Selection Method
Negative
Application
Cell Isolation
Brand
RosetteSep
Area of Interest
Cancer Research, Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36 | 15127, 15167 | All | English |
| Safety Data Sheet | RosetteSep™ CTC Enrichment Cocktail Containing Anti-CD36 | 15127, 15167 | All | English |
数据及文献
Data
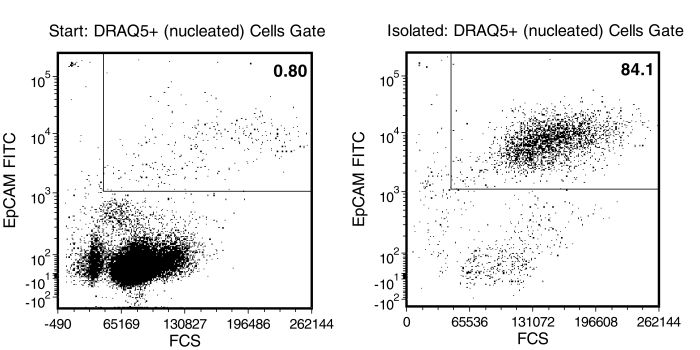
Figure 1. Typical RosetteSep™ CTC Enrichment Profile
In the example above, CAMA (epithelial tumor cell line) cells were seeded into whole blood at a starting frequency of 0.8%. The CAMA cell content of the enriched fraction is 84%. Typically 3.4 to 4.7 log depletion of targeted CD45+ cells is attained.
Publications (7)
Scientific reports 2020 jan
Establishment of novel long-term cultures from EpCAM positive and negative circulating tumour cells from patients with metastatic gastroesophageal cancer.
Abstract
Abstract
Circulating tumour cell (CTC) enumeration and profiling has been established as a valuable clinical tool in many solid malignancies. A key challenge in CTC research is the limited number of cells available for study. Ex vivo CTC culture permits expansion of these rare cell populations for detailed characterisation, functional assays including drug sensitivity testing, and investigation of the pathobiology of metastases. We report for the first time the establishment and characterisation of two continuous CTC lines from patients with gastroesophageal cancer. The two cell lines (designated UWG01CTC and UWG02CTC) demonstrated rapid tumorigenic growth in immunodeficient mice and exhibit distinct genotypic and phenotypic profiles which are consistent with the tumours of origin. UWG02CTC exhibits an EpCAM+, cytokeratin+, CD44+ phenotype, while UWG01CTC, which was derived from a patient with metastatic neuroendocrine cancer, displays an EpCAM-, weak cytokeratin phenotype, with strong expression of neuroendocrine markers. Further, the two cell lines show distinct differences in drug and radiation sensitivity which match differential cancer-associated gene expression pathways. This is strong evidence implicating EpCAM negative CTCs in metastasis. These novel, well characterised, long-term CTC cell lines from gastroesophageal cancer will facilitate ongoing research into metastasis and the discovery of therapeutic targets.
Seminars in cancer biology 2017 APR
How to study and overcome tumor heterogeneity with circulating biomarkers: The breast cancer case.
Abstract
Abstract
Breast cancer ranks first among female cancer-related deaths in Western countries. As the primary tumor can often be controlled by surgical resection, the survival of women with breast cancer is closely linked to the incidence of distant metastases. Molecular screening by next generation sequencing highlighted the spatial and temporal heterogeneity of solid tumors as well as the clonal evolution of cancer cells during progression and under treatment pressure. Such findings question whether an optimal assessment of disease progression and a screening for druggable mutations should be based on molecular features of primary or recurrent/metastatic lesions and therefore represent a crucial element for failure or success of personalized medicine. In fact, new targeted therapies may induce only short-term benefit annulled by the emergence of resistant clones with new driver mutations which would need to be rapidly and reliably identified. Serial tissue sampling is therefore essential but, unfortunately, also represents a problem since biopsies from solid lesions, which are invasive and potentially painful and risky, cannot be easily repeatedly sampled, are inaccessible or may not fully reflect tumor heterogeneity. The need to early detect and strike this moving target" is now directing the scientific community towards liquid biopsy-based biomarkers which include circulating tumor cells (CTC) and cell-free circulating tumor DNA (ctDNA) can be repeatedly assessed through non-invasive and easy-to-perform procedures and may act as reliable read-outs of functional and molecular features of recurrent/metastatic lesions. In this review we summarize the outcome of CTCs and ctDNA in breast cancer with special reference on their role on unveiling and overcoming tumor heterogeneity on their potential relevance for tumor surveillance and monitoring and for the selection of therapeutic options. Finally we propose integration between blood-based molecular and clinical approaches for monitoring disease progression according to the specific pattern of recurrence of the most aggressive breast cancer molecular subtypes."
Scientific reports 2016 DEC
Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients.
Abstract
Abstract
The relevance of blood-based assays to monitor minimal residual disease (MRD) in non-metastatic prostate cancer (PCa) remains unclear. Proving that clinically relevant circulating tumor cells (CTCs) can be detected with available technologies could address this. This study aimed to improve CTC detection in non-metastatic PCa patients by combining three independent CTC assays: the CellSearch system, an in vivo CellCollector and the EPISPOT. Peripheral blood samples from high-risk PCa patients were screened for CTCs before and three months after radical prostatectomy (RP). Combining the results of both time points, CTCs were detected in 37{\%}, 54.9{\%} and 58.7{\%} of patients using CellSearch, CellCollector and EPISPOT, respectively. The cumulative positivity rate of the three CTC assays was 81.3{\%} (87/107) with 21.5{\%} (23/107) of patients harboring ≥5 CTCs/7.5 ml blood. Matched pair analysis of 30 blood samples taken before and after surgery indicated a significant decrease in CTCs captured by the CellCollector from 66{\%} before RP to 34{\%} after therapy (p = 0.031). CTC detection by EPISPOT before RP significantly correlated with PSA serum values (p {\textless} 0.0001) and clinical tumor stage (p = 0.04), while the other assays showed no significant correlations. In conclusion, CTC-based liquid biopsies have the potential to monitor MRD in patients with non-metastatic prostate cancer.
Nature medicine 2014 AUG
Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer.
Abstract
Abstract
Small-cell lung cancer (SCLC), an aggressive neuroendocrine tumor with early dissemination and dismal prognosis, accounts for 15-20{\%} of lung cancer cases and ∼200,000 deaths each year. Most cases are inoperable, and biopsies to investigate SCLC biology are rarely obtainable. Circulating tumor cells (CTCs), which are prevalent in SCLC, present a readily accessible 'liquid biopsy'. Here we show that CTCs from patients with either chemosensitive or chemorefractory SCLC are tumorigenic in immune-compromised mice, and the resultant CTC-derived explants (CDXs) mirror the donor patient's response to platinum and etoposide chemotherapy. Genomic analysis of isolated CTCs revealed considerable similarity to the corresponding CDX. Most marked differences were observed between CDXs from patients with different clinical outcomes. These data demonstrate that CTC molecular analysis via serial blood sampling could facilitate delivery of personalized medicine for SCLC. CDXs are readily passaged, and these unique mouse models provide tractable systems for therapy testing and understanding drug resistance mechanisms.
British medical bulletin 2010 JAN
New technologies for the detection of circulating tumour cells.
Abstract
Abstract
The vast majority of cancer-related death is due to the metastatic spread of the primary tumour. Circulating tumour cells (CTC) are essential for establishing metastasis and their detection has long been considered as a possible tool to assess the aggressiveness of a given tumour and its potential of subsequent growth at distant organs. Conventional markers are not reliable in detecting occult metastasis and, for example, fail to identify approximately 40% of cancer patients in need of more aggressive or better adjusted therapies. Recent studies in metastatic breast cancer have shown that CTC detection can be used as a marker for overall survival and assessment of the therapeutic response. The benefits of CTC detection in early breast cancer and other solid tumours need further validation. Moreover, optimal CTC detection techniques are the subject of controversy as several lack reproducibility, sensitivity and/or specificity. Recent technical advances allow CTC detection and characterization at the single-cell level in the blood or in the bone marrow. Their reproducibility propels the use of CTC in cancer staging and real-time monitoring of systemic anticancer therapies in several large clinical trials. CTC assays are being integrated in large clinical trials to establish their potential in the management of cancer patients and improve our understanding of metastasis biology. This review will focus on the techniques currently used, the technical advancements made, the limitations of CTC detection and future perspectives in this field.
Clinical cancer research : an official journal of the American Association for Cancer Research 2009 FEB
Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer.
Abstract
Abstract
PURPOSE: Circulating cell-free DNA in the blood of cancer patients harbors tumor-specific aberrations. Here, we investigated whether this DNA might also reflect the presence of circulating tumor cells (CTC). EXPERIMENTAL DESIGN: To identify the source of cell-free DNA in blood, plasma derived from 81 patients with prostate cancer was examined for CTCs and cell-free DNA. An epithelial immunospot assay was applied for detection of CTCs, and a PCR-based fluorescence microsatellite analysis with a panel of 14 polymorphic markers was used for detection of allelic imbalances (AI). RESULTS: The plasma DNA levels significantly correlated with the diagnosis subgroups of localized (stage M0, n = 69) and metastasized prostate cancer (stage M1, n = 12; P = 0.03) and with the tumor stage of these patients (P textless 0.005). AI was found on cell-free DNA in plasma from 45.0% and 58.5% of M0 and M1 patients, respectively. Detection of CTCs showed that 71.0% or 92.0% of the M0 and M1 patients harbored 1 to 40 CTCs in their blood, respectively. The occurrence of CTCs correlated with tumor stage (P textless 0.03) and increasing Gleason scores (P = 0.04). Notably, significant associations of the number of CTCs with the AI frequencies at the markers D8S137 (P = 0.03), D9S171 (P = 0.04), and D17S855 (P = 0.02) encoding the cytoskeletal protein dematin, the inhibitor of the cyclin-dependent kinase CDKN2/p16 and BRCA1, respectively, were observed. CONCLUSIONS: These findings show, for the first time, a relationship between the occurrence of CTCs and circulating tumor-associated DNA in blood, which, therefore, might become a valuable new source for monitoring metastatic progression in cancer patients.




