EasySep™ Mouse SCA1 Positive Selection Kit
Immunomagnetic positive selection kit
概要
The EasySep™ Mouse SCA1 Positive Selection Kit is designed to isolate SCA1+ cells from single-cell suspensions of bone marrow by positive selection. The cocktail contains a combination of PE-labeled antibodies that is directed against SCA1, which are then recognized by antibody complexes that are directed against PE and dextran. Labeled cells are bound to magnetic particles and separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off.
This kit replaces the EasySep™ Mouse SCA1 Biotin Positive Selection Kit (Catalog #18856) for even faster cell isolations.
This kit replaces the EasySep™ Mouse SCA1 Biotin Positive Selection Kit (Catalog #18856) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 97% purity
• No columns required
• Up to 97% purity
• No columns required
Components
- EasySep™ Mouse SCA1 Positive Selection Kit (Catalog #18756)
- EasySep™ Mouse SCA1 PE Labeling Reagent, 1 mL
- EasySep™ PE Positive Selection Cocktail, 2 x 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Mouse SCA1 Positive Selection Kit with Filter Tips (Catalog #18756RF)
- EasySep™ Mouse SCA1 PE Labeling Reagent, 1 mL
- EasySep™ PE Positive Selection Cocktail, 2 x 1 mL
- EasySep™ Dextran RapidSpheres™, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125) x 2
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Mouse
Sample Source
Bone Marrow
Selection Method
Positive
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse SCA1 Positive Selection Kit | 18756 | All | English |
| Product Information Sheet | RoboSep™ Mouse SCA1 Positive Selection Kit with Filter Tips | 18756RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse SCA1 Positive Selection Kit | 18756 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse SCA1 Positive Selection Kit | 18756 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse SCA1 Positive Selection Kit with Filter Tips | 18756RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse SCA1 Positive Selection Kit with Filter Tips | 18756RF | All | English |
数据及文献
Data
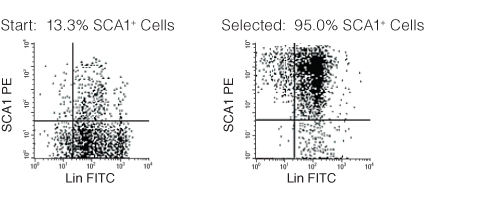
Figure 1. FACS Profile Results with EasySep™ Mouse SCA1 Positive Selection Kit
Starting with mouse bone marrow, the SCA1+ cell content of the selected cells typically ranges from 87 - 97%.
Publications (4)
Aging Cell 2019 aug
Long‐term repopulation of aged bone marrow stem cells using young Sca‐1 cells promotes aged heart rejuvenation
Abstract
Abstract
Reduced quantity and quality of stem cells in aged individuals hinders cardiac repair and regeneration after injury. We used young bone marrow (BM) stem cell antigen 1 (Sca-1) cells to reconstitute aged BM and rejuvenate the aged heart, and examined the underlying molecular mechanisms. BM Sca-1+ or Sca-1- cells from young (2-3 months) or aged (18-19 months) GFP transgenic mice were transplanted into lethally irradiated aged mice to generate 4 groups of chimeras: young Sca-1+ , young Sca-1- , old Sca-1+ , and old Sca-1- . Four months later, expression of rejuvenation-related genes (Bmi1, Cbx8, PNUTS, Sirt1, Sirt2, Sirt6) and proteins (CDK2, CDK4) was increased along with telomerase activity and telomerase-related protein (DNA-PKcs, TRF-2) expression, whereas expression of senescence-related genes (p16INK4a , P19ARF , p27Kip1 ) and proteins (p16INK4a , p27Kip1 ) was decreased in Sca-1+ chimeric hearts, especially in the young group. Host cardiac endothelial cells (GFP- CD31+ ) but not cardiomyocytes were the primary cell type rejuvenated by young Sca-1+ cells as shown by improved proliferation, migration, and tubular formation abilities. C-X-C chemokine CXCL12 was the factor most highly expressed in homed donor BM (GFP+ ) cells isolated from young Sca-1+ chimeric hearts. Protein expression of Cxcr4, phospho-Akt, and phospho-FoxO3a in endothelial cells derived from the aged chimeric heart was increased, especially in the young Sca-1+ group. Reconstitution of aged BM with young Sca-1+ cells resulted in effective homing of functional stem cells in the aged heart. These young, regenerative stem cells promoted aged heart rejuvenation through activation of the Cxcl12/Cxcr4 pathway of cardiac endothelial cells.
Arteriosclerosis, thrombosis, and vascular biology 2008 AUG
Lentiviral transduction of apoAI into hematopoietic progenitor cells and macrophages: applications to cell therapy of atherosclerosis.
Abstract
Abstract
OBJECTIVE: We used genetically engineered mouse hematopoietic progenitor cells (HPCs) to investigate the therapeutic effects of human apoAI on atherosclerosis in apoE(-/-) mice. METHODS AND RESULTS: Lentiviral constructs expressing either human apoAI (LV-apoAI) or green fluorescent protein (LV-GFP) cDNA under a macrophage specific promoter (CD68) were generated and used for ex vivo transduction of mouse HPCs and macrophages. The transduction efficiency was textgreater25% for HPCs and textgreater70% for macrophages. ApoAI was found in the macrophage culture media, mostly associated with the HDL fraction. Interestingly, a significant increase in mRNA and protein levels for ATP binding cassette A1 (ABCA1) and ABCG1 were found in apoAI-expressing macrophages after acLDL loading. Expression of apoAI significantly increased cholesterol efflux in wild-type and apoE(-/-) macrophages. HPCs transduced with LV-apoAI ex vivo and then transplanted into apoE(-/-) mice caused a 50% reduction in atherosclerotic lesion area compared to GFP controls, without influencing plasma HDL-C levels. CONCLUSIONS: Lentiviral transduction of apoAI into HPCs reduces atherosclerosis in apoE(-/-) mice. Expression of apoAI in macrophages improves cholesterol trafficking in wild-type apoE-producing macrophages and causes upregulation of ABCA1 and ABCG1. These novel observations set the stage for a cell therapy approach to atherosclerosis regression, exploiting the cooperation between apoE and apoAI to maximize cholesterol exit from the plaque.
Blood 2006 NOV
Low SCL/TAL1 expression reveals its major role in adult hematopoietic myeloid progenitors and stem cells.
Abstract
Abstract
Stem cell leukemia/T cell acute leukemia 1 (SCL/TAL1) plays a key role in the development of murine primitive hematopoiesis but its functions in adult definitive hematopoiesis are still unclear. Using lentiviral delivery of TAL1-directed shRNA in human hematopoietic cells, we show that decreased expression of TAL1 induced major disorders at different levels of adult hematopoietic cell development. Erythroid and myeloid cell production in cultures was dramatically decreased in TAL1-directed shRNA-expressing cells, whereas lymphoid B-cell development was normal. These results confirm the role of TAL1 in the erythroid compartment and show TLA1's implication in the function of myeloid committed progenitors. Moreover, long-term cultures and transplantation of TAL1-directed shRNA-expressing CD34+ cells into irradiated nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice led to dramatically low levels of human cells of all lineages including the B-lymphoid lineage, strongly suggesting that TAL1 has a role in the early commitment of hematopoietic stem cells (HSCs) in humans. Cultures and transplantation experiments performed with mouse Sca1+ cells gave identical results. Altogether, these observations definitively show that TAL1 participates in the regulation of hematopoiesis from HSCs to myeloid progenitors, and pinpoint TAL1 as a master protein of human and murine adult hematopoiesis.
Blood 2004 JUN
ABC transporter activities of murine hematopoietic stem cells vary according to their developmental and activation status.
Abstract
Abstract
Primitive hematopoietic cells from several species are known to efflux both Hoechst 33342 and Rhodamine-123. We now show that murine hematopoietic stem cells (HSCs) defined by long-term multilineage repopulation assays efflux both dyes variably according to their developmental or activation status. In day 14.5 murine fetal liver, very few HSCs efflux Hoechst 33342 efficiently, and they are thus not detected as side population" (SP) cells. HSCs in mouse fetal liver also fail to efflux Rhodamine-123. Both of these features are retained by most of the HSCs present until 4 weeks after birth but are reversed by 8 weeks of age or after a new HSC population is regenerated in adult mice that receive transplants with murine fetal liver cells. Activation of adult HSCs in vivo following 5-fluorouracil treatment�