EasySep™ Mouse NK Cell Isolation Kit
25-Minute cell isolation kit using immunomagnetic negative selection
概要
The EasySep™ Mouse NK Cell Isolation Kit targets non-NK cells by labeling unwanted cells with biotinylated antibodies and EasySep™ Streptavidin RapidSpheres™, and separates cells without columns using an EasySep™ magnet. Desired cells are simply poured off into a new tube. Isolated cells are immediately available for downstream applications such as flow cytometry, culture or cell-based assays.
This product replaces the EasySep™ Mouse NK Cell Enrichment Kit (Catalog #19755) for even faster cell isolations.
This product replaces the EasySep™ Mouse NK Cell Enrichment Kit (Catalog #19755) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• No columns required
• Untouched, viable cells
• No columns required
• Untouched, viable cells
Components
- EasySep™ Mouse NK Cell Isolation Kit (Catalog #19855)
- EasySep™ Mouse NK Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50002, 1 mL
- RoboSep™ Mouse NK Cell Isolation Kit (Catalog #19855RF)
- EasySep™ Mouse NK Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50002, 1 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
NK Cells
Species
Mouse
Sample Source
Spleen
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse NK Cell Isolation Kit | 19855 | All | English |
| Product Information Sheet | RoboSep™ Mouse NK Cell Isolation Kit | 19855RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse NK Cell Isolation Kit | 19855 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse NK Cell Isolation Kit | 19855 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse NK Cell Isolation Kit | 19855RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse NK Cell Isolation Kit | 19855RF | All | English |
数据及文献
Data
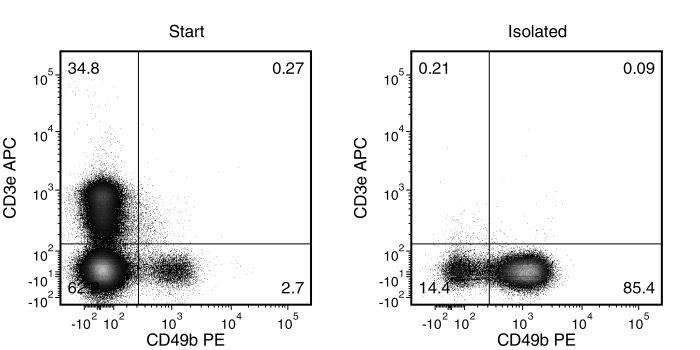
Figure 1. Typical EasySep™ Mouse NK Cell Isolation Profile
Starting with mouse splenocytes, the NK cell (CD3-CD49b+) content of the isolated fraction typically ranges from 67 - 89%.
Publications (8)
Cell reports 2020 may
CRISPR-Cas9 Ribonucleoprotein-Mediated Genomic Editing in Mature Primary Innate Immune Cells.
Abstract
Abstract
CRISPR genome engineering has become a powerful tool to functionally investigate the complex mechanisms of immune system regulation. While decades of work have aimed to genetically reprogram innate immunity, the utility of current approaches is restricted by poor knockout efficiencies or limited specificity for mature cell lineages in vivo. Here, we describe an optimized strategy for non-viral CRISPR-Cas9 ribonucleoprotein (cRNP) genomic editing of mature primary mouse innate lymphocyte cells (ILCs) and myeloid lineage cells that results in an almost complete loss of single or double target gene expression from a single electroporation. Furthermore, we describe in vivo adoptive transfer mouse models that can be utilized to screen for gene function during viral infection using cRNP-edited naive natural killer (NK) cells and bone-marrow-derived conventional dendritic cell precursors (cDCPs). This resource will enhance target gene discovery and offer a specific and simplified approach to gene editing in the mouse innate immune system.
Journal of immunology (Baltimore, Md. : 1950) 2019 jun
NK Cells Are Critical for Optimal Immunity to Experimental Trypanosoma congolense Infection.
Abstract
Abstract
NK cells are key innate immune cells that play critical roles in host defense. Although NK cells have been shown to regulate immunity to some infectious diseases, their role in immunity to Trypanosoma congolense has not been investigated. NK cells are vital sources of IFN-gamma and TNF-alpha; two key cytokines that are known to play important roles in resistance to African trypanosomes. In this article, we show that infection with T. congolense leads to increased levels of activated and functional NK cells in multiple tissue compartments. Systemic depletion of NK cells with anti-NK1.1 mAb led to increased parasitemia, which was accompanied by significant reduction in IFN-gamma production by immune cells in the spleens and liver of infected mice. Strikingly, infected NFIL3-/- mice (which genetically lack NK cell development and function) on the normally resistant background were highly susceptible to T. congolense infection. These mice developed fulminating and uncontrolled parasitemia and died significantly earlier (13 ± 1 d) than their wild-type control mice (106 ± 26 d). The enhanced susceptibility of NFIL3-/- mice to infection was accompanied by significantly impaired cytokine (IFN-gamma and TNF-alpha) response by CD3+ T cells in the spleens and liver. Adoptive transfer of NK cells into NFIL3-/- mice before infection rescued them from acute death in a perforin-dependent manner. Collectively, these studies show that NK cells are critical for optimal resistance to T. congolense, and its deficiency leads to enhanced susceptibility in infected mice.
Nature nanotechnology 2019 jan
Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy.
Abstract
Abstract
Cyclic dinucleotide (CDN) agonists of stimulator of interferon genes (STING) are a promising class of immunotherapeutics that activate innate immunity to increase tumour immunogenicity. However, the efficacy of CDNs is limited by drug delivery barriers, including poor cellular targeting, rapid clearance and inefficient transport to the cytosol where STING is localized. Here, we describe STING-activating nanoparticles (STING-NPs)-rationally designed polymersomes for enhanced cytosolic delivery of the endogenous CDN ligand for STING, 2'3' cyclic guanosine monophosphate-adenosine monophosphate (cGAMP). STING-NPs increase the biological potency of cGAMP, enhance STING signalling in the tumour microenvironment and sentinel lymph node, and convert immunosuppressive tumours to immunogenic, tumoricidal microenvironments. This leads to enhanced therapeutic efficacy of cGAMP, inhibition of tumour growth, increased rates of long-term survival, improved response to immune checkpoint blockade and induction of immunological memory that protects against tumour rechallenge. We validate STING-NPs in freshly isolated human melanoma tissue, highlighting their potential to improve clinical outcomes of immunotherapy.
Cell stem cell 2019 dec
PRDM16 Maintains Homeostasis of the Intestinal Epithelium by Controlling Region-Specific Metabolism.
Abstract
Abstract
Metabolic pathways dynamically regulate tissue development and maintenance. However, the mechanisms that govern the metabolic adaptation of stem or progenitor cells to their local niche are poorly understood. Here, we define the transcription factor PRDM16 as a region-specific regulator of intestinal metabolism and epithelial renewal. PRDM16 is selectively expressed in the upper intestine, with enrichment in crypt-resident progenitor cells. Acute Prdm16 deletion in mice triggered progenitor apoptosis, leading to diminished epithelial differentiation and severe intestinal atrophy. Genomic and metabolic analyses showed that PRDM16 transcriptionally controls fatty acid oxidation (FAO) in crypts. Expression of this PRDM16-driven FAO program was highest in the upper small intestine and declined distally. Accordingly, deletion of Prdm16 or inhibition of FAO selectively impaired the development and maintenance of upper intestinal enteroids, and these effects were rescued by acetate treatment. Collectively, these data reveal that regionally specified metabolic programs regulate intestinal maintenance.
Nature 2019
Immunity to commensal papillomaviruses protects against skin cancer.
Abstract
Abstract
Immunosuppression increases the risk of cancers that are associated with viral infection1. In particular, the risk of squamous cell carcinoma of the skin-which has been associated with beta human papillomavirus ($\beta$-HPV) infection-is increased by more than 100-fold in immunosuppressed patients2-4. Previous studies have not established a causative role for HPVs in driving the development of skin cancer. Here we show that T cell immunity against commensal papillomaviruses suppresses skin cancer in immunocompetent hosts, and the loss of this immunity-rather than the oncogenic effect of HPVs-causes the markedly increased risk of skin cancer in immunosuppressed patients. To investigate the effects of papillomavirus on carcinogen-driven skin cancer, we colonized several strains of immunocompetent mice with mouse papillomavirus type 1 (MmuPV1)5. Mice with natural immunity against MmuPV1 after colonization and acquired immunity through the transfer of T cells from immune mice or by MmuPV1 vaccination were protected against skin carcinogenesis induced by chemicals or by ultraviolet radiation in a manner dependent on CD8+ T cells. RNA and DNA in situ hybridization probes for 25 commensal $\beta$-HPVs revealed a significant reduction in viral activity and load in human skin cancer compared with the adjacent healthy skin, suggesting a strong immune selection against virus-positive malignant cells. Consistently, E7 peptides from $\beta$-HPVs activated CD8+ T cells from unaffected human skin. Our findings reveal a beneficial role for commensal viruses and establish a foundation for immune-based approaches that could block the development of skin cancer by boosting immunity against the commensal HPVs present in all of our skin.
Proceedings of the National Academy of Sciences of the United States of America 2018 NOV
CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1.
Abstract
Abstract
Natural killer (NK) cell recognition of tumor cells is mediated through activating receptors such as CD226, with suppression of effector functions often controlled by negative regulatory transcription factors such as FOXO1. Here we show that CD226 regulation of NK cell cytotoxicity is facilitated through inactivation of FOXO1. Gene-expression analysis of NK cells isolated from syngeneic tumors grown in wild-type or CD226-deficient mice revealed dysregulated expression of FOXO1-regulated genes in the absence of CD226. In vitro cytotoxicity and stimulation assays demonstrated that CD226 is required for optimal killing of tumor target cells, with engagement of its ligand CD155 resulting in phosphorylation of FOXO1. CD226 deficiency or anti-CD226 antibody blockade impaired cytotoxicity with concomitant compromised inactivation of FOXO1. Furthermore, inhibitors of FOXO1 phosphorylation abrogated CD226-mediated signaling and effector responses. These results define a pathway by which CD226 exerts control of NK cell responses against tumors.