EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit
Immunomagnetic negative selection kit
概要
The EasySep™ Mouse Hematopoietic Cell Isolation Kit is designed to isolate stem and progenitor cells from single-cell suspensions of bone marrow or other tissues by negative selection. Unwanted cells are targeted for removal with biotinylated antibodies directed against non-hematopoietic stem cells and non-progenitor cells (CD5, CD11b, CD19, CD45R/B220, Ly6G/C(Gr-1), TER119, 7-4) and streptavidin-coated magnetic particles (RapidSpheres™ ). Labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
This product replaces the EasySep™ Mouse Hematopoietic Progenitor Cell Enrichment Kit (Catalog #19756) for faster cell isolations.
This product replaces the EasySep™ Mouse Hematopoietic Progenitor Cell Enrichment Kit (Catalog #19756) for faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 84% purity
• No columns required
• Untouched, viable cells
• Up to 84% purity
• No columns required
• Untouched, viable cells
Components
- EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit (Catalog #19856)
- EasySep™ Mouse Hematopoietic Progenitor Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 1 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Mouse Hematopoietic Progenitor Cell Isolation Kit (Catalog #19856RF)
- EasySep™ Mouse Hematopoietic Progenitor Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 1 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Mouse
Sample Source
Bone Marrow
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856 | All | English |
| Product Information Sheet | RoboSep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | 19856RF | All | English |
数据及文献
Data
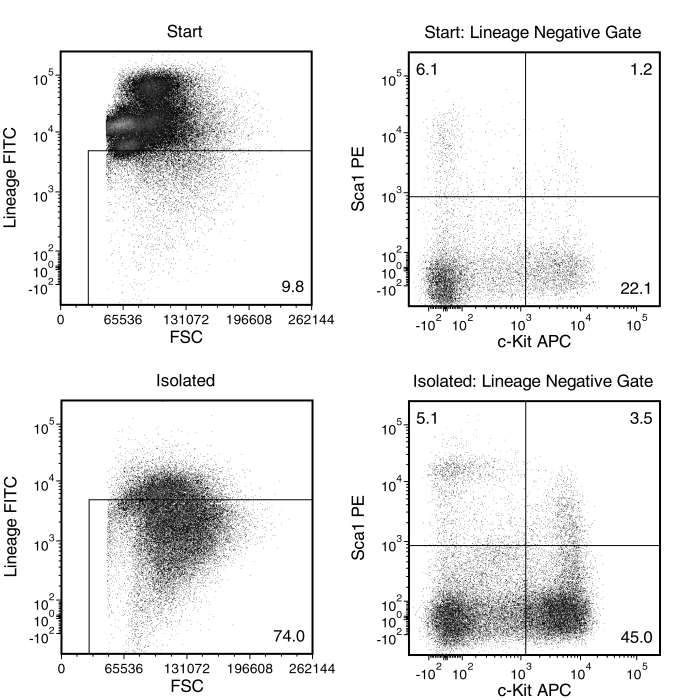
Figure 1. Typical EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Profile
Starting with a mouse bone marrow cell suspension, the lineage antigen-negative cell content of the isolated fraction typically ranges from 60 - 84%.
Publications (6)
Frontiers in immunology 2020
Dendritic Cell-Restricted Progenitors Contribute to Obesity-Associated Airway Inflammation via Adam17-p38 MAPK-Dependent Pathway.
Abstract
Abstract
Proliferation of dendritic cell (DC)-restricted progenitor cells in bone marrow compartment is tightly regulated at steady state and responds to multiple tissue-specific triggers during disturbed homeostasis such as obesity. DCs in the lung stem from a rapidly dividing DC-restricted progenitor cells and are effective at generating adaptive immune responses in allergic airway inflammation. Precisely, how DC-restricted progenitor expansion and differentiation are influenced by airway inflammation to maintain constant supply of myeloid DCs is poorly understood. Here we show that a high fat diet (HFD) induces oxidative stress and accelerates the expansion of DC- restricted progenitor cells in bone marrow and correlates with persistent induction of p38 mitogen activated protein kinase (MAPK), which is blocked with a selective p38$\alpha$/$\beta$ MAPK inhibitor. Mice fed a HFD and sensitized to inhaled allergen house dust mite (HDM) led to alterations of DC- restricted progenitor cells that were characterized by increased expansion and seeding of lung DCs in airway inflammation. Mechanistically, we establish that the expansion induced by HFD dysregulates the expression of a disintegrin and metallopeptidase domain 17 (Adam17) and is required for p38 MAPK activation in DC-restricted progenitors. These results demonstrates that obesity produces persistent changes in DC precursors and that elevation of Adam17 expression is tightly coupled to p38 MAPK and is a key driver of proliferation. Altogether, these data provide phenotypic and mechanistic insight into dendritic cell supply chain in obesity-associated airway inflammation.
Cell stem cell 2020
Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency.
Abstract
Abstract
Quiescence is a fundamental property that maintains hematopoietic stem cell (HSC) potency throughout life. Quiescent HSCs are thought to rely on glycolysis for their energy, but the overall metabolic properties of HSCs remain elusive. Using combined approaches, including single-cell RNA sequencing (RNA-seq), we show that mitochondrial membrane potential (MMP) distinguishes quiescent from cycling-primed HSCs. We found that primed, but not quiescent, HSCs relied readily on glycolysis. Notably, in vivo inhibition of glycolysis enhanced the competitive repopulation ability of primed HSCs. We further show that HSC quiescence is maintained by an abundance of large lysosomes. Repression of lysosomal activation in HSCs led to further enlargement of lysosomes while suppressing glucose uptake. This also induced increased lysosomal sequestration of mitochondria and enhanced the competitive repopulation ability of primed HSCs by over 90-fold in vivo. These findings show that restraining lysosomal activity preserves HSC quiescence and potency and may be therapeutically relevant.
Nature genetics 2019
Genomic subtyping and therapeutic targeting of acute erythroleukemia.
Abstract
Abstract
Acute erythroid leukemia (AEL) is a high-risk leukemia of poorly understood genetic basis, with controversy regarding diagnosis in the spectrum of myelodysplasia and myeloid leukemia. We compared genomic features of 159 childhood and adult AEL cases with non-AEL myeloid disorders and defined five age-related subgroups with distinct transcriptional profiles: adult, TP53 mutated; NPM1 mutated; KMT2A mutated/rearranged; adult, DDX41 mutated; and pediatric, NUP98 rearranged. Genomic features influenced outcome, with NPM1 mutations and HOXB9 overexpression being associated with a favorable prognosis and TP53, FLT3 or RB1 alterations associated with poor survival. Targetable signaling mutations were present in 45{\%} of cases and included recurrent mutations of ALK and NTRK1, the latter of which drives erythroid leukemogenesis sensitive to TRK inhibition. This genomic landscape of AEL provides the framework for accurate diagnosis and risk stratification of this disease, and the rationale for testing targeted therapies in this high-risk leukemia.
PLoS biology 2019
Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy.
Abstract
Abstract
Decidua is a transient uterine tissue shared by mammals with hemochorial placenta and is essential for pregnancy. The decidua is infiltrated by many immune cells promoting pregnancy. Adult bone marrow (BM)-derived cells (BMDCs) differentiate into rare populations of nonhematopoietic endometrial cells in the uterus. However, whether adult BMDCs become nonhematopoietic decidual cells and contribute functionally to pregnancy is unknown. Here, we show that pregnancy mobilizes mesenchymal stem cells (MSCs) to the circulation and that pregnancy induces considerable adult BMDCs recruitment to decidua, where some differentiate into nonhematopoietic prolactin-expressing decidual cells. To explore the functional importance of nonhematopoietic BMDCs to pregnancy, we used Homeobox a11 (Hoxa11)-deficient mice, having endometrial stromal-specific defects precluding decidualization and successful pregnancy. Hoxa11 expression in BM is restricted to nonhematopoietic cells. BM transplant (BMT) from wild-type (WT) to Hoxa11-/- mice results in stromal expansion, gland formation, and marked decidualization otherwise absent in Hoxa11-/- mice. Moreover, in Hoxa11+/- mice, which have increased pregnancy losses, BMT from WT donors leads to normalized uterine expression of numerous decidualization-related genes and rescue of pregnancy loss. Collectively, these findings reveal that adult BMDCs have a previously unrecognized nonhematopoietic physiologic contribution to decidual stroma, thereby playing important roles in decidualization and pregnancy.
Cell reports 2018 dec
A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome.
Abstract
Abstract
Molecular- and cellular-based therapies have the potential to reduce obesity-associated disease. In response to cold, beige adipocytes form in subcutaneous white adipose tissue and convert energy stored in metabolic substrates to heat, making them an attractive therapeutic target. We developed a robust method to generate a renewable source of human beige adipocytes from induced pluripotent stem cells (iPSCs). Developmentally, these cells are derived from FOXF1+ mesoderm and progress through an expandable mural-like mesenchymal stem cell (MSC) to form mature beige adipocytes that display a thermogenically active profile. This includes expression of uncoupling protein 1 (UCP1) concomitant with increased uncoupled respiration. With this method, dysfunctional adipogenic precursors can be reprogrammed and differentiated into beige adipocytes with increased thermogenic function and anti-diabetic secretion potential. This resource can be used to (1) elucidate mechanisms that underlie the control of beige adipogenesis and (2) generate material for cellular-based therapies that target metabolic syndrome in humans.
Cell stem cell 2018 dec
Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis.
Abstract
Abstract
Inflammation is a risk factor for cancer development. Individuals with preleukemic TET2 mutations manifest clonal hematopoiesis and are at a higher risk of developing leukemia. How inflammatory signals influence the survival of preleukemic hematopoietic stem and progenitor cells (HSPCs) is unclear. We show a rapid increase in the frequency and absolute number of Tet2-KO mature myeloid cells and HSPCs in response to inflammatory stress, which results in enhanced production of inflammatory cytokines, including interleukin-6 (IL-6), and resistance to apoptosis. IL-6 induces hyperactivation of the Shp2-Stat3 signaling axis, resulting in increased expression of a novel anti-apoptotic long non-coding RNA (lncRNAs), Morrbid, in Tet2-KO myeloid cells and HSPCs. Expression of activated Shp2 in HSPCs phenocopies Tet2 loss with regard to hyperactivation of Stat3 and Morrbid. In vivo, pharmacologic inhibition of Shp2 or Stat3 or genetic loss of Morrbid in Tet2 mutant mice rescues inflammatory-stress-induced abnormalities in HSPCs and mature myeloid cells, including clonal hematopoiesis.