EasySep™ Mouse CD8+ T Cell Isolation Kit
17.5-Minute cell isolation kit using immunomagnetic negative selection
概要
The EasySep™ Mouse CD8+ T Cell Isolation Kit is designed to isolate CD8+ T cells from single-cell suspensions of splenocytes or other tissues by negative selection. Unwanted cells are targeted for removal with biotinylated antibodies directed against non-CD8+ T cells and streptavidin-coated magnetic particles (RapidSpheres™ ). Labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
This product replaces the EasySep™ Mouse CD8+ T Cell Enrichment Kit (Catalog #19753) for even faster cell isolations.
This product replaces the EasySep™ Mouse CD8+ T Cell Enrichment Kit (Catalog #19753) for even faster cell isolations.
Advantages
• Fast and easy-to-use
• Up to 95% purity
• No columns required
• Untouched, viable cells
• Up to 95% purity
• No columns required
• Untouched, viable cells
Components
- EasySep™ Mouse CD8+ T Cell Isolation Kit (Catalog #19853)
- EasySep™ Mouse CD8+ T Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 2 x 1 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Mouse CD8+ T Cell Isolation Kit (Catalog #19853RF)
- EasySep™ Mouse CD8+ T Cell Isolation Cocktail, 0.5 mL
- EasySep™ Streptavidin RapidSpheres™ 50001, 2 x 1 mL
- Normal Rat Serum, 2 mL
- RoboSep™ Buffer (Catalog #20104)
- RoboSep™ Filter Tips (Catalog #20125)
Magnet Compatibility
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyPlate™ EasySep™ Magnet (Catalog 18102)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• RoboSep™-S (Catalog #21000)
Subtype
Cell Isolation Kits
Cell Type
T Cells, T Cells, CD8+
Species
Mouse
Sample Source
Other, Spleen
Selection Method
Negative
Application
Cell Isolation
Brand
EasySep, RoboSep
Area of Interest
Immunology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Mouse CD8+ T Cell Isolation Kit | 19853 | All | English |
| Product Information Sheet | RoboSep™ Mouse CD8+ T Cell Isolation Kit | 19853RF | All | English |
| Safety Data Sheet 1 | EasySep™ Mouse CD8+ T Cell Isolation Kit | 19853 | All | English |
| Safety Data Sheet 2 | EasySep™ Mouse CD8+ T Cell Isolation Kit | 19853 | All | English |
| Safety Data Sheet 3 | EasySep™ Mouse CD8+ T Cell Isolation Kit | 19853 | All | English |
| Safety Data Sheet 1 | RoboSep™ Mouse CD8+ T Cell Isolation Kit | 19853RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Mouse CD8+ T Cell Isolation Kit | 19853RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Mouse CD8+ T Cell Isolation Kit | 19853RF | All | English |
数据及文献
Data
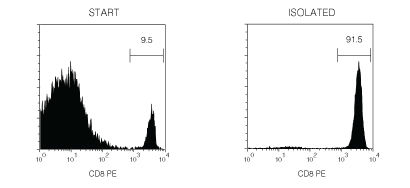
Figure 1. Typical EasySep™ Mouse CD8+ T Cell Isolation Profile
Starting with mouse splenocytes, the CD8+ T cell content of the isolated fraction typically ranges from 87 - 95%.
Publications (32)
Cell 2020 nov
Detecting Tumor Antigen-Specific T Cells via Interaction-Dependent Fucosyl-Biotinylation.
Abstract
Abstract
Re-activation and clonal expansion of tumor-specific antigen (TSA)-reactive T cells are critical to the success of checkpoint blockade and adoptive transfer of tumor-infiltrating lymphocyte (TIL)-based therapies. There are no reliable markers to specifically identify the repertoire of TSA-reactive T cells due to their heterogeneous composition. We introduce FucoID as a general platform to detect endogenous antigen-specific T cells for studying their biology. Through this interaction-dependent labeling approach, intratumoral TSA-reactive CD4+, CD8+ T cells, and TSA-suppressive CD4+ T cells can be detected and separated from bystander T cells based on their cell-surface enzymatic fucosyl-biotinylation. Compared to bystander TILs, TSA-reactive TILs possess a distinct T cell receptor (TCR) repertoire and unique gene features. Although exhibiting a dysfunctional phenotype, TSA-reactive CD8+ TILs possess substantial capabilities of proliferation and tumor-specific killing. Featuring genetic manipulation-free procedures and a quick turnover cycle, FucoID should have the potential of accelerating the pace of personalized cancer treatment.
Nature biomedical engineering 2020 jun
Pharmacokinetic tuning of protein-antigen fusions enhances the immunogenicity of T-cell vaccines.
Abstract
Abstract
The formulations of peptide-based antitumour vaccines being tested in clinical studies are generally associated with weak potency. Here, we show that pharmacokinetically tuning the responses of peptide vaccines by fusing the peptide epitopes to carrier proteins optimizes vaccine immunogenicity in mice. In particular, we show in immunized mice that the carrier protein transthyretin simultaneously optimizes three factors: efficient antigen uptake in draining lymphatics from the site of injection, protection of antigen payloads from proteolytic degradation and reduction of antigen presentation in uninflamed distal lymphoid organs. Optimizing these factors increases vaccine immunogenicity by up to 90-fold and maximizes the responses to viral antigens, tumour-associated antigens, oncofetal antigens and shared neoantigens. Protein-peptide epitope fusions represent a facile and generalizable strategy for enhancing the T-cell responses elicited by subunit vaccines.
Nature communications 2020 jan
Lymphatic endothelial cells prime na\ive CD8+ T cells into memory cells under steady-state conditions."
Abstract
Abstract
Lymphatic endothelial cells (LECs) chemoattract na{\{i}}ve T cells and promote their survival in the lymph nodes and can cross-present antigens to na{\"{i}}ve CD8+ T cells to drive their proliferation despite lacking key costimulatory molecules. However the functional consequence of LEC priming of CD8+ T cells is unknown. Here we show that while many proliferating LEC-educated T cells enter early apoptosis the remainders comprise a long-lived memory subset with transcriptional metabolic and phenotypic features of central memory and stem cell-like memory T cells. In vivo these memory cells preferentially home to lymph nodes and display rapid proliferation and effector differentiation following memory recall and can protect mice against a subsequent bacterial infection. These findings introduce a new immunomodulatory role for LECs in directly generating a memory-like subset of quiescent yet antigen-experienced CD8+ T cells that are long-lived and can rapidly differentiate into effector cells upon inflammatory antigenic challenge."""
Cell metabolism 2020 jan
STAT3 Activation-Induced Fatty Acid Oxidation in CD8+ T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth.
Abstract
Abstract
Although obesity is known to be critical for cancer development, how obesity negatively impacts antitumor immune responses remains largely unknown. Here, we show that increased fatty acid oxidation (FAO) driven by activated STAT3 in CD8+ T effector cells is critical for obesity-associated breast tumor progression. Ablating T cell Stat3 or treatment with an FAO inhibitor in obese mice spontaneously developing breast tumor reduces FAO, increases glycolysis and CD8+ T effector cell functions, leading to inhibition of breast tumor development. Moreover, PD-1 ligation in CD8+ T cells activates STAT3 to increase FAO, inhibiting CD8+ T effector cell glycolysis and functions. Finally, leptin enriched in mammary adipocytes and fat tissues downregulates CD8+ T cell effector functions through activating STAT3-FAO and inhibiting glycolysis. We identify a critical role of increased oxidation of fatty acids driven by leptin and PD-1 through STAT3 in inhibiting CD8+ T effector cell glycolysis and in promoting obesity-associated breast tumorigenesis.
Nature communications 2020
PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade.
Abstract
Abstract
Immune checkpoint blockade therapies have shown clinical promise in a variety of cancers, but how tumor-infiltrating T cells are activated remains unclear. In this study, we explore the functions of PD-L1 on dendritic cells (DCs), which highly express PD-L1. We observe that PD-L1 on DC plays a critical role in limiting T cell responses. Type 1 conventional DCs are essential for PD-L1 blockade and they upregulate PD-L1 upon antigen uptake. Upregulation of PD-L1 on DC is mediated by type II interferon. While DCs are the major antigen presenting cells for cross-presenting tumor antigens to T cells, subsequent PD-L1 upregulation protects them from killing by cytotoxic T lymphocytes, yet dampens the antitumor responses. Blocking PD-L1 in established tumors promotes re-activation of tumor-infiltrating T cells for tumor control. Our study identifies a critical and dynamic role of PD-L1 on DC, which needs to be harnessed for better invigoration of antitumor immune responses.
Nature biomedical engineering 2020
Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours.
Abstract
Abstract
Checkpoint-inhibitor (CPI) immunotherapy has achieved remarkable clinical success, yet its efficacy in 'immunologically cold' tumours has been modest. Interleukin-12 (IL-12) is a powerful cytokine that activates the innate and adaptive arms of the immune system; however, the administration of IL-12 has been associated with immune-related adverse events. Here we show that, after intravenous administration of a collagen-binding domain fused to IL-12 (CBD-IL-12) in mice bearing aggressive mouse tumours, CBD-IL-12 accumulates in the tumour stroma due to exposed collagen in the disordered tumour vasculature. In comparison with the administration of unmodified IL-12, CBD-IL-12 induced sustained intratumoural levels of interferon-$\gamma$, substantially reduced its systemic levels as well as organ damage and provided superior anticancer efficacy, eliciting complete regression of CPI-unresponsive breast tumours. Furthermore, CBD-IL-12 potently synergized with CPI to eradicate large established melanomas, induced antigen-specific immunological memory and controlled tumour growth in a genetically engineered mouse model of melanoma. CBD-IL-12 may potentiate CPI immunotherapy for immunologically cold tumours.