NeuroCult™ Differentiation Supplement (Mouse & Rat)
Supplement for differentiation of mouse and rat neural stem and progenitor cells
概要
NeuroCult™ Differentiation Supplement (Mouse & Rat) is a standardized, serum-containing supplement for the differentiation of mouse and rat neural stem and progenitor cells into astrocytes, neurons, and oligodendrocytes when used in combination with NeuroCult™ Basal Medium (Mouse & Rat; Catalog #05700). This supplement is also a component of NeuroCult™ Differentiation Kit (Mouse & Rat; Catalog #05704).
Contains
Serum
Subtype
Supplements
Cell Type
Brain Tumor Stem Cells, Neural Stem and Progenitor Cells
Species
Mouse, Rat
Application
Cell Culture, Differentiation, Functional Assay
Brand
NeuroCult
Area of Interest
Neuroscience, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | NeuroCult™ Differentiation Supplement (Mouse & Rat) | 05703 | All | English |
| Manual | NeuroCult™ Differentiation Supplement (Mouse & Rat) | 05703 | All | English |
| Safety Data Sheet | NeuroCult™ Differentiation Supplement (Mouse & Rat) | 05703 | All | English |
数据及文献
Data
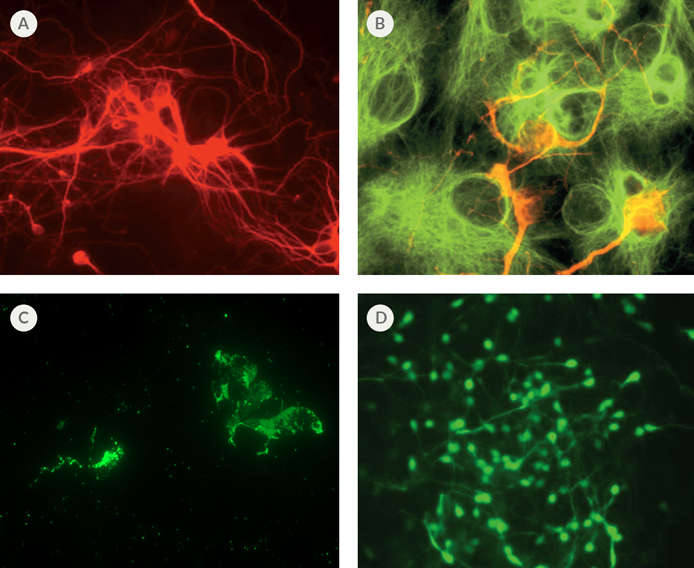
Figure 1. Differentiation of Mouse Neural Stem Cells Using NeuroCult™
(A) Immunofluorescent staining of neural cell body and processes (red) with mouse monoclonal ß-Tubulin III antibody. (B) Immunofluorescent staining of astrocytes (green) with rabbit polyclonal GFAP antibody and neurons (red) with mouse monoclonal MAP2 antibody. (C) Immunofluorescent staining of oligodendrocytes (green) with mouse monoclonal O4 Oligodendrocyte Marker antibody. (D) Immunofluorescent staining of GABA-nergic neurons (green) with rabbit polyclonal GABA antibody.
Publications (26)
Neuroscience Letters 2017 MAR
Recombinant insulin-like growth factor binding protein-4 inhibits proliferation and promotes differentiation of neural progenitor cells
Abstract
Abstract
Insulin-like growth factor (IGF) is involved in regulating many processes during neural development, and IGF binding protein-4 (IGFBP4) functions as a modulator of IGF actions or in an IGF-independent manner (e.g., via inhibiting Wnt/β-catenin signaling). In the present study, neural progenitor cells (NPCs) were isolated from the forebrain of newborn mice to investigate effects of IGFBP4 on the proliferation and differentiation of NPCs. The proliferation of NPCs was evaluated using Cell Counting Kit-8 (CCK-8) after treatment with or without IGFBP4 as well as blockers of IGF-IR and β-catenin. Phosphorylation levels of Akt, Erk1, 2 and p38 were analyzed by Western blotting. The differentiation of NPCs was evaluated using immunofluorescence and Western blotting. It was shown that exogenous IGFBP4 significantly inhibited the proliferation of NPCs and it did not induce a more pronounced inhibition of cell proliferation after blockade of IGF-IR but it did after antagonism of β-catenin. Akt phosphorylation was significantly decreased and phosphorylation levels of Erk1, 2 and p38 were not significantly changed in IGFBP4-treated NPCs. Excessive IGFBP4 significantly promoted NPCs to differentiate into astrocytes and neurons. These data suggested that exogenous IGFBP4 inhibits proliferation and promotes differentiation of neural progenitor cells mainly through IGF-IR signaling pathway.
Molecular and Cellular Biology 2017 JAN
L2hgdh deficiency accumulates L-2-hydroxyglutarate with progressive leukoencephalopathy and neurodegeneration
Abstract
Abstract
L-2-hydroxyglutarate aciduria (L-2-HGA) is an autosomal recessive neurometabolic disorder caused by a mutation in the L-2-hydroxyglutarate dehydrogenase ( L2HGDH ) gene. In this study, we generated L2hgdh knockout (KO) mice and observed a robust increase of 2-hydroxyglutarate (L-2-HG) levels in multiple tissues. The highest levels of L-2-HG were observed in the brain and testis with a corresponding increase in histone methylation in these tissues. L2hgdh KO mice exhibit white matter abnormalities, extensive gliosis, microglia-mediated neuroinflammation, and an expansion of oligodendrocyte progenitor cells (OPCs). Moreover, L2hgdh deficiency leads to impaired adult hippocampal neurogenesis and late-onset neurodegeneration in mouse brains. Our data provide in vivo evidence that L2hgdh mutation leads to L-2-HG accumulation, leukoencephalopathy, and neurodegeneration in mice, thus offering new insights into the pathophysiology of L-2-HGA in humans.
Scientific reports 2016 MAY
Generation and gene expression profiling of 48 transcription-factor-inducible mouse embryonic stem cell lines.
Abstract
Abstract
Mouse embryonic stem cells (ESCs) can differentiate into a wide range - and possibly all cell types in vitro, and thus provide an ideal platform to study systematically the action of transcription factors (TFs) in cell differentiation. Previously, we have generated and analyzed 137 TF-inducible mouse ESC lines. As an extension of this NIA Mouse ESC Bank we generated and characterized 48 additional mouse ESC lines, in which single TFs in each line could be induced in a doxycycline-controllable manner. Together, with the previous ESC lines, the bank now comprises 185 TF-manipulable ESC lines (>10% of all mouse TFs). Global gene expression (transcriptome) profiling revealed that the induction of individual TFs in mouse ESCs for 48 hours shifts their transcriptomes toward specific differentiation fates (e.g., neural lineages by Myt1 Isl1, and St18; mesodermal lineages by Pitx1, Pitx2, Barhl2, and Lmx1a; white blood cells by Myb, Etv2, and Tbx6, and ovary by Pitx1, Pitx2, and Dmrtc2). These data also provide and lists of inferred target genes of each TF and possible functions of these TFs. The results demonstrate the utility of mouse ESC lines and their transcriptome data for understanding the mechanism of cell differentiation and the function of TFs.
International Immunopharmacology 2014 MAY
Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma
Abstract
Abstract
New therapeutic strategies are needed in the treatment of asthma besides vaccines and pharmacotherapies. For the development of novel therapies, the use of mesenchymal stem cells (MSCs) is a promising approach in regenerative medicine. Delivery of compact bone (CB) derived MSCs to the injured lungs is an alternative treatment strategy for chronic asthma. In this study, we aimed to isolate highly enriched population of MSCs from mouse CB with regenerative capacity, and to investigate the impact of these cells in airway remodeling and inflammation in experimental ovalbumin-induced mouse model of chronic asthma. mCB-MSCs were isolated, characterized, labeled with GFP and then transferred into mice with chronic asthma developed by ovalbumin (OVA) provocation. Histopathological changes including basement membrane, epithelium, subepithelial smooth thickness and goblet cell hyperplasia, and MSCs migration to lung tissues were evaluated. These histopathological alterations were increased in ovalbumin-treated mice compared to PBS group (P<0.001). Intravenous administration of mCB-MSC significantly reduced these histopathological changes in both distal and proximal airways (P<0.001). We showed that GFP-labeled MSCs were located in the lungs of OVA group 2weeks after intravenous induction. mCB-MSCs also significantly promoted Treg response in ovalbumin-treated mice (OVA+MSC group) (P<0.037). Our studies revealed that mCB-MSCs migrated to lung tissue and suppressed histopathological changes in murine model of asthma. The results reported here provided evidence that mCB-MSCs may be an alternative strategy for the treatment of remodeling and inflammation associated with chronic asthma.
Toxicology in Vitro 2014 JUN
In vitro detection of cytotoxicity using FluoroJade-C
Abstract
Abstract
We describe here a novel method for the determination of cytotoxicity in cell cultures using Fluoro-Jade C (FJ-C). FJ-C has been previously used for the assessment of neurodegeneration in fixed brain tissue samples, and has never been utilized in live cell cultures or in different types of cells other than neurons. In the present study we examined the utility of FJ-C for the determination of cytotoxicity in vitro. Various cell cultures were evaluated including neural stem cells, brain microvessel endothelial cells, and SH-SY5Y, PC12 and MDCK cells. Cytotoxicities induced by toxicants in cell cultures, as determined by the FJ-C labeling, were further confirmed by commonly used cytotoxicity assays. This in vitro approach is simple, fast, and sensitive and, thus, has the potential to augment if not replace currently used cell-based cytotoxicity assays.
Brain Research 2014 JAN
Expression of the CHOP-inducible carbonic anhydrase CAVI-b is required for BDNF-mediated protection from hypoxia
Abstract
Abstract
Carbonic anhydrases (CAs) comprise a family of zinc-containing enzymes that catalyze the reversible hydration of carbon dioxide. CAs contribute to a myriad of physiological processes, including pH regulation, anion transport and water balance. To date, 16 known members of the mammalian alpha-CA family have been identified. Given that the catalytic family members share identical reaction chemistry, their physiologic roles are influenced greatly by their tissue and sub-cellular locations. CAVI is the lone secreted CA and exists in both saliva and the gastrointestinal mucosa. An alternative, stress-inducible isoform of CAVI (CAVI-b) has been shown to be expressed from a cryptic promoter that is activated by the CCAAT/Enhancer-Binding Protein Homologous Protein (CHOP). The CAVI-b isoform is not secreted and is currently of unknown physiological function. Here we use neuronal models, including a model derived using Car6 and CHOP gene ablations, to delineate a role for CAVI-b in ischemic protection. Our results demonstrate that CAVI-b expression, which is increased through CHOP-signaling in response to unfolded protein stress, is also increased by oxygen-glucose deprivation (OGD). While enforced expression of CAVI-b is not sufficient to protect against ischemia, CHOP regulation of CAVI-b is necessary for adaptive changes mediated by BDNF that reduce subsequent ischemic damage. These results suggest that CAVI-b comprises a necessary component of a larger adaptive signaling pathway downstream of CHOP.