NeuroCult™ Differentiation Kit (Mouse & Rat)
Medium kit for differentiation of mouse and rat neural stem and progenitor cells
概要
NeuroCult™ Differentiation Kit (Mouse & Rat) is a standardized medium and supplement kit for the differentiation of mouse and rat neural stem and progenitor cells into neurons, astrocytes, and oligodendrocytes.
Components
- NeuroCult™ Basal Medium (Mouse & Rat), 450 mL (Catalog #05700)
- NeuroCult™ Differentiation Supplement (Mouse & Rat), 50 mL (Catalog #05703)
Contains
Serum
Subtype
Specialized Media
Cell Type
Brain Tumor Stem Cells, Neural Stem and Progenitor Cells
Species
Mouse, Rat
Application
Cell Culture, Differentiation, Functional Assay
Brand
NeuroCult
Area of Interest
Neuroscience, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | NeuroCult™ Differentiation Kit (Mouse & Rat) | 05704 | All | English |
| Product Information Sheet 2 | NeuroCult™ Differentiation Kit (Mouse & Rat) | 05704 | All | English |
| Manual | NeuroCult™ Differentiation Kit (Mouse & Rat) | 05704 | All | English |
| Safety Data Sheet | NeuroCult™ Differentiation Kit (Mouse & Rat) | 05704 | All | English |
数据及文献
Data
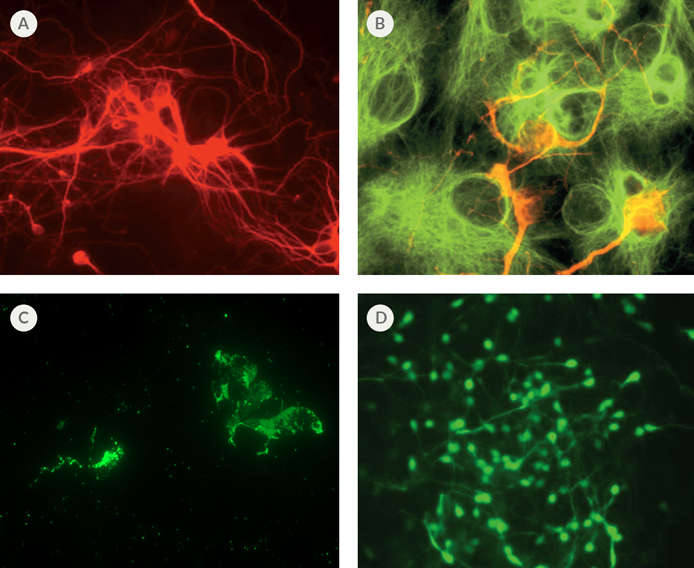
Figure 1. Differentiation of Mouse Neural Stem Cells Using NeuroCult™
(A) Immunofluorescent staining of neural cell body and processes (red) with mouse monoclonal ß-Tubulin III antibody. (B) Immunofluorescent staining of astrocytes (green) with rabbit polyclonal GFAP antibody and neurons (red) with mouse monoclonal MAP2 antibody. (C) Immunofluorescent staining of oligodendrocytes (green) with mouse monoclonal O4 Oligodendrocyte Marker antibody. (D) Immunofluorescent staining of GABA-nergic neurons (green) with rabbit polyclonal GABA antibody.
Publications (30)
Neuroscience Letters 2017 MAR
Recombinant insulin-like growth factor binding protein-4 inhibits proliferation and promotes differentiation of neural progenitor cells
Abstract
Abstract
Insulin-like growth factor (IGF) is involved in regulating many processes during neural development, and IGF binding protein-4 (IGFBP4) functions as a modulator of IGF actions or in an IGF-independent manner (e.g., via inhibiting Wnt/β-catenin signaling). In the present study, neural progenitor cells (NPCs) were isolated from the forebrain of newborn mice to investigate effects of IGFBP4 on the proliferation and differentiation of NPCs. The proliferation of NPCs was evaluated using Cell Counting Kit-8 (CCK-8) after treatment with or without IGFBP4 as well as blockers of IGF-IR and β-catenin. Phosphorylation levels of Akt, Erk1, 2 and p38 were analyzed by Western blotting. The differentiation of NPCs was evaluated using immunofluorescence and Western blotting. It was shown that exogenous IGFBP4 significantly inhibited the proliferation of NPCs and it did not induce a more pronounced inhibition of cell proliferation after blockade of IGF-IR but it did after antagonism of β-catenin. Akt phosphorylation was significantly decreased and phosphorylation levels of Erk1, 2 and p38 were not significantly changed in IGFBP4-treated NPCs. Excessive IGFBP4 significantly promoted NPCs to differentiate into astrocytes and neurons. These data suggested that exogenous IGFBP4 inhibits proliferation and promotes differentiation of neural progenitor cells mainly through IGF-IR signaling pathway.
Molecular and Cellular Biology 2017 JAN
L2hgdh deficiency accumulates L-2-hydroxyglutarate with progressive leukoencephalopathy and neurodegeneration
Abstract
Abstract
L-2-hydroxyglutarate aciduria (L-2-HGA) is an autosomal recessive neurometabolic disorder caused by a mutation in the L-2-hydroxyglutarate dehydrogenase ( L2HGDH ) gene. In this study, we generated L2hgdh knockout (KO) mice and observed a robust increase of 2-hydroxyglutarate (L-2-HG) levels in multiple tissues. The highest levels of L-2-HG were observed in the brain and testis with a corresponding increase in histone methylation in these tissues. L2hgdh KO mice exhibit white matter abnormalities, extensive gliosis, microglia-mediated neuroinflammation, and an expansion of oligodendrocyte progenitor cells (OPCs). Moreover, L2hgdh deficiency leads to impaired adult hippocampal neurogenesis and late-onset neurodegeneration in mouse brains. Our data provide in vivo evidence that L2hgdh mutation leads to L-2-HG accumulation, leukoencephalopathy, and neurodegeneration in mice, thus offering new insights into the pathophysiology of L-2-HGA in humans.
Scientific reports 2016 SEP
Exercise protects against methamphetamine-induced aberrant neurogenesis.
Abstract
Abstract
While no effective therapy is available for the treatment of methamphetamine (METH)-induced neurotoxicity, aerobic exercise is being proposed to improve depressive symptoms and substance abuse outcomes. The present study focuses on the effect of exercise on METH-induced aberrant neurogenesis in the hippocampal dentate gyrus in the context of the blood-brain barrier (BBB) pathology. Mice were administered with METH or saline by i.p. injections for 5 days with an escalating dose regimen. One set of mice was sacrificed 24 h post last injection of METH, and the remaining animals were either subjected to voluntary wheel running (exercised mice) or remained in sedentary housing (sedentary mice). METH administration decreased expression of tight junction (TJ) proteins and increased BBB permeability in the hippocampus. These changes were preserved post METH administration in sedentary mice and were associated with the development of significant aberrations of neural differentiation. Exercise protected against these effects by enhancing the protein expression of TJ proteins, stabilizing the BBB integrity, and enhancing the neural differentiation. In addition, exercise protected against METH-induced systemic increase in inflammatory cytokine levels. These results suggest that exercise can attenuate METH-induced neurotoxicity by protecting against the BBB disruption and related microenvironmental changes in the hippocampus.
In vitro cellular & developmental biology. Animal 2016 OCT
Induction of specific neuron types by overexpression of single transcription factors.
Abstract
Abstract
Specific neuronal types derived from embryonic stem cells (ESCs) can facilitate mechanistic studies and potentially aid in regenerative medicine. Existing induction methods, however, mostly rely on the effects of the combined action of multiple added growth factors, which generally tend to result in mixed populations of neurons. Here, we report that overexpression of specific transcription factors (TFs) in ESCs can rather guide the differentiation of ESCs towards specific neuron lineages. Analysis of data on gene expression changes 2 d after induction of each of 185 TFs implicated candidate TFs for further ESC differentiation studies. Induction of 23 TFs (out of 49 TFs tested) for 6 d facilitated neural differentiation of ESCs as inferred from increased proportion of cells with neural progenitor marker PSA-NCAM. We identified early activation of the Notch signaling pathway as a common feature of most potent inducers of neural differentiation. The majority of neuron-like cells generated by induction of Ascl1, Smad7, Nr2f1, Dlx2, Dlx4, Nr2f2, Barhl2, and Lhx1 were GABA-positive and expressed other markers of GABAergic neurons. In the same way, we identified Lmx1a and Nr4a2 as inducers for neurons bearing dopaminergic markers and Isl1, Fezf2, and St18 for cholinergic motor neurons. A time-course experiment with induction of Ascl1 showed early upregulation of most neural-specific messenger RNA (mRNA) and microRNAs (miRNAs). Sets of Ascl1-induced mRNAs and miRNAs were enriched in Ascl1 targets. In further studies, enrichment of cells obtained with the induction of Ascl1, Smad7, and Nr2f1 using microbeads resulted in essentially pure population of neuron-like cells with expression profiles similar to neural tissues and expressed markers of GABAergic neurons. In summary, this study indicates that induction of transcription factors is a promising approach to generate cultures that show the transcription profiles characteristic of specific neural cell types.
Scientific reports 2016 MAY
Generation and gene expression profiling of 48 transcription-factor-inducible mouse embryonic stem cell lines.
Abstract
Abstract
Mouse embryonic stem cells (ESCs) can differentiate into a wide range - and possibly all cell types in vitro, and thus provide an ideal platform to study systematically the action of transcription factors (TFs) in cell differentiation. Previously, we have generated and analyzed 137 TF-inducible mouse ESC lines. As an extension of this NIA Mouse ESC Bank we generated and characterized 48 additional mouse ESC lines, in which single TFs in each line could be induced in a doxycycline-controllable manner. Together, with the previous ESC lines, the bank now comprises 185 TF-manipulable ESC lines (>10% of all mouse TFs). Global gene expression (transcriptome) profiling revealed that the induction of individual TFs in mouse ESCs for 48 hours shifts their transcriptomes toward specific differentiation fates (e.g., neural lineages by Myt1 Isl1, and St18; mesodermal lineages by Pitx1, Pitx2, Barhl2, and Lmx1a; white blood cells by Myb, Etv2, and Tbx6, and ovary by Pitx1, Pitx2, and Dmrtc2). These data also provide and lists of inferred target genes of each TF and possible functions of these TFs. The results demonstrate the utility of mouse ESC lines and their transcriptome data for understanding the mechanism of cell differentiation and the function of TFs.
Biomaterials 2016 MAY
Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo.
Abstract
Abstract
Cell therapy offers an innovative approach for treating enteric neuropathies. Postnatal gut-derived enteric neural stem/progenitor cells (ENSCs) represent a potential autologous source, but have a limited capacity for proliferation and neuronal differentiation. Since serotonin (5-HT) promotes enteric neuronal growth during embryonic development, we hypothesized that serotonin receptor agonism would augment growth of neurons from transplanted ENSCs. Postnatal ENSCs were isolated from 2 to 4 week-old mouse colon and cultured with 5-HT4 receptor agonist (RS67506)-loaded liposomal nanoparticles. ENSCs were co-cultured with mouse colon explants in the presence of RS67506-loaded (n = 3) or empty nanoparticles (n = 3). ENSCs were also transplanted into mouse rectum in vivo with RS67506-loaded (n = 8) or blank nanoparticles (n = 4) confined in a thermosensitive hydrogel, Pluronic F-127. Neuronal density and proliferation were analyzed immunohistochemically. Cultured ENSCs gave rise to significantly more neurons in the presence of RS67506-loaded nanoparticles. Similarly, colon explants had significantly increased neuronal density when RS67506-loaded nanoparticles were present. Finally, following in vivo cell delivery, co-transplantation of ENSCs with 5-HT4 receptor agonist-loaded nanoparticles led to significantly increased neuronal density and proliferation. We conclude that optimization of postnatal ENSCs can support their use in cell-based therapies for neurointestinal diseases.