MyeloCult™ H5100
Medium for long-term culture of human cells
概要
MyeloCult™ H5100 is a culture medium for the initiation and maintenance of myeloid long-term cultures of human hematopoietic cells and stromal cell feeder layers. The sera used in this formulation have been pre-tested and selected for their ability to support long-term myelopoiesis by primitive human hematopoietic cells (e.g. in long-term culture-initiating cell (LTC-IC) assays).
MyeloCult™ H5100 requires the addition of freshly prepared Hydrocortisone (Catalog #07904).
MyeloCult™ H5100 requires the addition of freshly prepared Hydrocortisone (Catalog #07904).
Contains
• Horse serum
• Fetal bovine serum
• 2-Mercaptoethanol
• Minimum Essential Medium (MEM) Alpha
• Supplements
• Fetal bovine serum
• 2-Mercaptoethanol
• Minimum Essential Medium (MEM) Alpha
• Supplements
Subtype
Specialized Media
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Human
Application
Cell Culture, Functional Assay
Brand
MyeloCult
Area of Interest
Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | MyeloCult™ H5100 | 05150 | All | English |
| Product Information Sheet 2 | MyeloCult™ H5100 | 05150 | All | English |
| Manual | MyeloCult™ H5100 | 05100 | All | English |
| Safety Data Sheet | MyeloCult™ H5100 | 05150 | All | English |
数据及文献
Data
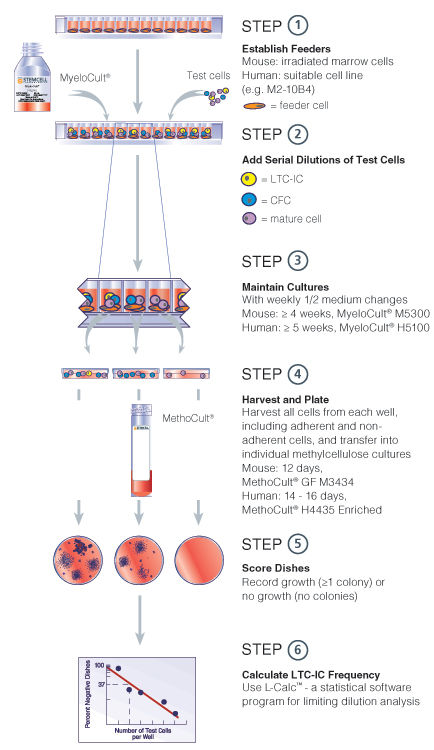
Figure 1. Limiting dilution LTC-IC assay
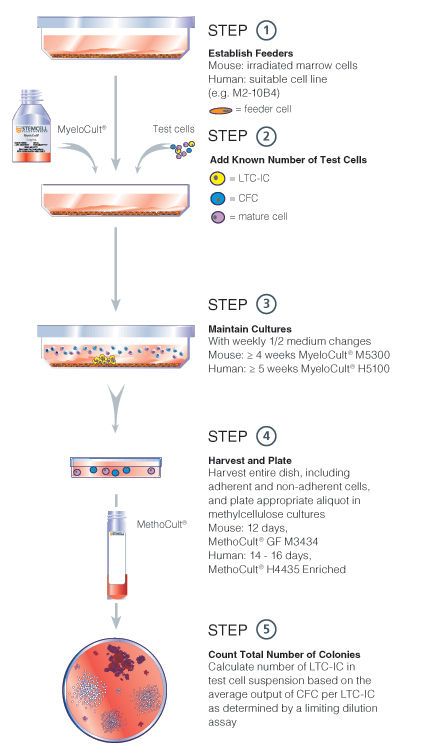
Figure 2. Bulk culture LTC-IC assay
Publications (61)
Nature communications 2016
Human NK cell development requires CD56-mediated motility and formation of the developmental synapse.
Abstract
Abstract
While distinct stages of natural killer (NK) cell development have been defined, the molecular interactions that shape human NK cell maturation are poorly understood. Here we define intercellular interactions between developing NK cells and stromal cells which, through contact-dependent mechanisms, promote the generation of mature, functional human NK cells from CD34(+) precursors. We show that developing NK cells undergo unique, developmental stage-specific sustained and transient interactions with developmentally supportive stromal cells, and that the relative motility of NK cells increases as they move through development in vitro and ex vivo. These interactions include the formation of a synapse between developing NK cells and stromal cells, which we term the developmental synapse. Finally, we identify a role for CD56 in developmental synapse structure, NK cell motility and NK cell development. Thus, we define the developmental synapse leading to human NK cell functional maturation.
Blood 2010 JAN
The AC133+CD38-, but not the rhodamine-low, phenotype tracks LTC-IC and SRC function in human cord blood ex vivo expansion cultures.
Abstract
Abstract
Phenotypic markers associated with human hematopoietic stem cells (HSCs) were developed and validated using uncultured cells. Because phenotype and function can be dissociated during culture, better markers to prospectively track and isolate HSCs in ex vivo cultures could be instrumental in advancing HSC-based therapies. Using an expansion system previously shown to increase hematopoietic progenitors and SCID-repopulating cells (SRCs), we demonstrated that the rhodamine-low phenotype was lost, whereas AC133 expression was retained throughout culture. Furthermore, the AC133(+)CD38(-) subpopulation was significantly enriched in long-term culture-initiating cells (LTC-IC) and SRCs after culture. Preculture and postculture analysis of total nucleated cell and LTC-IC number, and limiting dilution analysis in NOD/SCID mice, showed a 43-fold expansion of the AC133(+)CD38(-) subpopulation that corresponded to a 7.3-fold and 4.4-fold expansion of LTC-ICs and SRCs in this subpopulation, respectively. Thus, AC133(+)CD38(-) is an improved marker that tracks and enriches for LTC-IC and SRC in ex vivo cultures.
Stem cells (Dayton, Ohio) 2009 MAY
beta-Catenin expression in the bone marrow microenvironment is required for long-term maintenance of primitive hematopoietic cells.
Abstract
Abstract
Hematopoiesis is dependent upon the bone marrow microenvironment, which is comprised of multiple mesenchymal cell types, including fibroblasts, endothelial cells, osteoblasts, and stroma progenitors. The canonical Wnt signaling pathway, which relies on the beta-catenin protein to mediate its signal, is necessary for the normal development of mesenchymal tissue. We hypothesized that canonical Wnt signaling regulates the cellular composition and function of the bone marrow microenvironment. We observed that a beta-catenin-deficient bone marrow microenvironment maintained hematopoietic stem cells but exhibited a decreased capacity to support primitive hematopoietic cells. These results correlated with decreased numbers of osteoblasts and with decreased production of basic fibroblast growth factor, stem cell factor, and vascular cell adhesion molecule-1. From these data, we propose a model in which beta-catenin in the microenvironment is required noncell autonomously for long-term maintenance of hematopoietic progenitors.
Blood 2009 MAR
High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle.
Abstract
Abstract
Evidence suggests the transcription factor GATA-2 is a critical regulator of murine hematopoietic stem cells. Here, we explore the relation between GATA-2 and cell proliferation and show that inducing GATA-2 increases quiescence (G(0) residency) of murine and human hematopoietic cells. In human cord blood, quiescent fractions (CD34(+)CD38(-)Hoechst(lo)Pyronin Y(lo)) express more GATA-2 than cycling counterparts. Enforcing GATA-2 expression increased quiescence of cord blood cells, reducing proliferation and performance in long-term culture-initiating cell and colony-forming cell (CFC) assays. Gene expression analysis places GATA-2 upstream of the quiescence regulator MEF, but enforcing MEF expression does not prevent GATA-2-conferred quiescence, suggesting additional regulators are involved. Although known quiescence regulators p21(CIP1) and p27(KIP1) do not appear to be responsible, enforcing GATA-2 reduced expression of regulators of cell cycle such as CCND3, CDK4, and CDK6. Enforcing GATA-2 inhibited human hematopoiesis in vivo: cells with highest exogenous expression (GATA-2(hi)) failed to contribute to hematopoiesis in nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice, whereas GATA-2(lo) cells contributed with delayed kinetics and low efficiency, with reduced expression of Ki-67. Thus, GATA-2 activity inhibits cell cycle in vitro and in vivo, highlighting GATA-2 as a molecular entry point into the transcriptional program regulating quiescence in human hematopoietic stem and progenitor cells.
Immunity 2008 OCT
Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk.
Abstract
Abstract
Many cellular responses, such as autoimmunity and cytotoxicity, are controlled by receptors with cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs). Here, we showed that binding of inhibitory natural killer (NK) cell receptors to human leukocyte antigen (HLA) class I on target cells induced tyrosine phosphorylation of the adaptor Crk, concomitant with dephosphorylation of the guanine exchange factor Vav1. Furthermore, Crk dissociated from the guanine exchange factor C3G and bound to the tyrosine kinase c-Abl during inhibition. Membrane targeting of a tyrosine-mutated form of Crk could overcome inhibition of NK cell cytotoxicity, providing functional evidence that Crk phosphorylation contributes to inhibition. The specific phosphorylation of Crk and its dissociation from a signaling complex, observed here with two types of inhibitory receptors, expands the signaling potential of the large ITIM-receptor family and reveals an unsuspected component of the inhibitory mechanism.
Stem cells (Dayton, Ohio) 2007 JAN
Placental growth factor-1 potentiates hematopoietic progenitor cell mobilization induced by granulocyte colony-stimulating factor in mice and nonhuman primates.
Abstract
Abstract
The complex hematopoietic effects of placental growth factor (PlGF) prompted us to test in mice and nonhuman primates the mobilization of peripheral blood progenitor cells (PBPCs) elicited by recombinant mouse PlGF-2 (rmPlGF-2) and recombinant human PlGF-1 (rhPlGF-1). PBPC mobilization was evaluated by assaying colony-forming cells (CFCs), high-proliferative potential-CFCs (HPP-CFCs), and long-term culture-initiating cells (LTC-ICs). In mice, both rmPlGF-2 and rhPlGF-1 used as single agents failed to mobilize PBPCs, whereas the combination of rhPlGF-1 and granulocyte colony-stimulating factor (rhG-CSF) increased CFCs and LTC-ICs per milliliter of blood by four- and eightfold, respectively, as compared with rhG-CSF alone. rhPlGF-1 plus rhG-CSF significantly increased matrix metalloproteinase-9 plasma levels over rhG-CSF alone, suggesting a mechanistic explanation for rhPlGF-1/rhG-CSF synergism. In rhesus monkeys, rhPlGF-1 alone had no mobilization effect, whereas rhPlGF-1 (260 microg/kg per day) plus rhG-CSF (100 microg/kg per day) increased rhG-CSF-elicited mobilization of CFCs, HPP-CFCs, and LTC-ICs per milliliter of blood by 5-, 7-, and 15-fold, respectively. No specific toxicity was associated with the administration of rhPlGF-1 alone or in combination. In conclusion, our data demonstrate that rhPlGF-1 significantly increases rhG-CSF-elicited hematopoietic mobilization and provide a preclinical rationale for evaluating rhPlGF-1 in the clinical setting.