MethoCult™ SF M3436
Serum-free methylcellulose-based medium with recombinant cytokines (including EPO) for mouse erythroid progenitor cells
概要
MethoCult™ SF M3436 is optimized for the growth and enumeration of primitive erythroid progenitor cells (BFU-E) in colony-forming unit (CFU) assays of mouse bone marrow, fetal liver, spleen, peripheral blood, and purified cells. MethoCult™ SF M3436 does not support the growth of granulocyte-macrophage progenitor cells (CFU-GM, CFU-G and CFU-M) or multipotential granulocyte, erythroid, macrophage, megakaryocyte progenitor cells (CFU-GEMM). This formulation is serum-free and compatible with STEMvision™ software for automated colony counting of mouse bone marrow CFU assays. MethoCult™ SF M3436 can also be used for growth and enumeration of BFU-E in CFU assays of rat bone marrow cells.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Contains
• Methylcellulose in Iscove's MDM
• Bovine serum albumin
• Recombinant human insulin
• Human transferrin (iron-saturated)
• 2-Mercaptoethanol
• Cytokines (including recombinant human erythropoietin [EPO])
• Supplements
• Bovine serum albumin
• Recombinant human insulin
• Human transferrin (iron-saturated)
• 2-Mercaptoethanol
• Cytokines (including recombinant human erythropoietin [EPO])
• Supplements
Subtype
Semi-Solid Media, Specialized Media
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Mouse, Rat
Application
Cell Culture, Colony Assay, Functional Assay
Brand
MethoCult
Area of Interest
Drug Discovery and Toxicity Testing, Stem Cell Biology
Formulation
Serum-Free
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MethoCult™ SF M3436 | 03436 | All | English |
| Manual | MethoCult™ SF M3436 | 03436 | All | English |
| Safety Data Sheet | MethoCult™ SF M3436 | 03436 | All | English |
数据及文献
Data
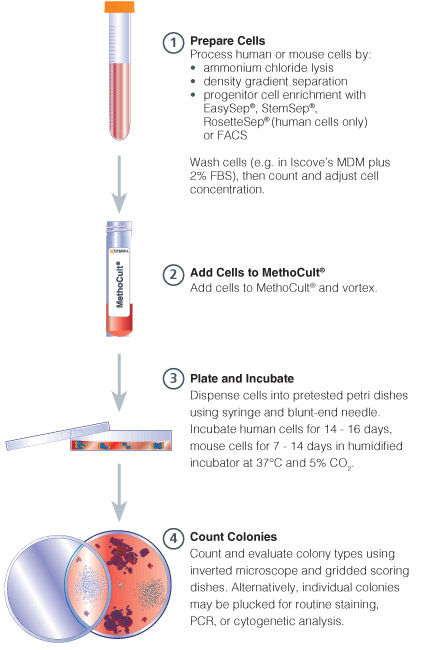
Figure 1. Procedure Summary for Hematopoietic CFU Assays
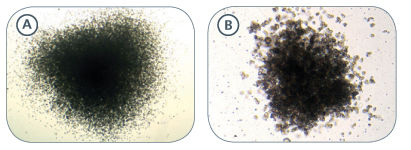
Figure 2. Examples of Colonies Derived From Mouse Hematopoietic Progenitors
BFU-E-derived colonies from mouse fetal liver (A) and spleen (B) samples cultured in MethoCult™ SF M3436
Publications (2)
Nature genetics 2019
Genomic subtyping and therapeutic targeting of acute erythroleukemia.
Abstract
Abstract
Acute erythroid leukemia (AEL) is a high-risk leukemia of poorly understood genetic basis, with controversy regarding diagnosis in the spectrum of myelodysplasia and myeloid leukemia. We compared genomic features of 159 childhood and adult AEL cases with non-AEL myeloid disorders and defined five age-related subgroups with distinct transcriptional profiles: adult, TP53 mutated; NPM1 mutated; KMT2A mutated/rearranged; adult, DDX41 mutated; and pediatric, NUP98 rearranged. Genomic features influenced outcome, with NPM1 mutations and HOXB9 overexpression being associated with a favorable prognosis and TP53, FLT3 or RB1 alterations associated with poor survival. Targetable signaling mutations were present in 45{\%} of cases and included recurrent mutations of ALK and NTRK1, the latter of which drives erythroid leukemogenesis sensitive to TRK inhibition. This genomic landscape of AEL provides the framework for accurate diagnosis and risk stratification of this disease, and the rationale for testing targeted therapies in this high-risk leukemia.
Blood 2008 OCT
Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS.
Abstract
Abstract
MDS is characterized by ineffective hematopoiesis that leads to peripheral cytopenias. Development of effective treatments has been impeded by limited insight into pathogenic pathways governing dysplastic growth of hematopoietic progenitors. We demonstrate that smad2, a downstream mediator of transforming growth factor-beta (TGF-beta) receptor I kinase (TBRI) activation, is constitutively activated in MDS bone marrow (BM) precursors and is overexpressed in gene expression profiles of MDS CD34(+) cells, providing direct evidence of overactivation of TGF-beta pathway in this disease. Suppression of the TGF-beta signaling by lentiviral shRNA-mediated down-regulation of TBRI leads to in vitro enhancement of hematopoiesis in MDS progenitors. Pharmacologic inhibition of TBRI (alk5) kinase by a small molecule inhibitor, SD-208, inhibits smad2 activation in hematopoietic progenitors, suppresses TGF-beta-mediated gene activation in BM stromal cells, and reverses TGF-beta-mediated cell-cycle arrest in BM CD34(+) cells. Furthermore, SD-208 treatment alleviates anemia and stimulates hematopoiesis in vivo in a novel murine model of bone marrow failure generated by constitutive hepatic expression of TGF-beta1. Moreover, in vitro pharmacologic inhibition of TBRI kinase leads to enhancement of hematopoiesis in varied morphologic MDS subtypes. These data directly implicate TGF-beta signaling in the pathobiology of ineffective hematopoiesis and identify TBRI as a potential therapeutic target in low-risk MDS.