MethoCult™ GF M3534
Methylcellulose-based medium with recombinant cytokines (without erythropoietin [EPO]) for mouse myeloid progenitor cells
概要
MethoCult™ GF M3534 is optimized for the growth and enumeration of granulocyte-macrophage progenitor cells (CFU-GM, CFU-G, CFU-M) in colony-forming unit (CFU) assays of mouse bone marrow, spleen, peripheral blood, and fetal liver cells. MethoCult™ M3534 does not support the growth of erythroid progenitor cells (BFU-E and CFU-E) as it does not contain erythropoietin (EPO). This formulation is compatible with STEMvision™ software for automated colony counting of mouse bone marrow CFU assays.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay.
Contains
• Methylcellulose in Iscove's MDM
• Fetal bovine serum
• Bovine serum albumin
• Recombinant human insulin
• Human transferrin (iron-saturated)
• 2-Mercaptoethanol
• Recombinant mouse stem cell factor (SCF)
• Recombinant mouse interleukin 3 (IL-3)
• Recombinant human interleukin 6 (IL-6)
• Supplements
• Fetal bovine serum
• Bovine serum albumin
• Recombinant human insulin
• Human transferrin (iron-saturated)
• 2-Mercaptoethanol
• Recombinant mouse stem cell factor (SCF)
• Recombinant mouse interleukin 3 (IL-3)
• Recombinant human interleukin 6 (IL-6)
• Supplements
Subtype
Semi-Solid Media, Specialized Media
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Mouse
Application
Cell Culture, Colony Assay, Functional Assay
Brand
MethoCult
Area of Interest
Drug Discovery and Toxicity Testing, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MethoCult™ GF M3534 | 03534 | All | English |
| Manual | MethoCult™ GF M3534 | 03534 | All | English |
| Safety Data Sheet | MethoCult™ GF M3534 | 03534 | All | English |
数据及文献
Data
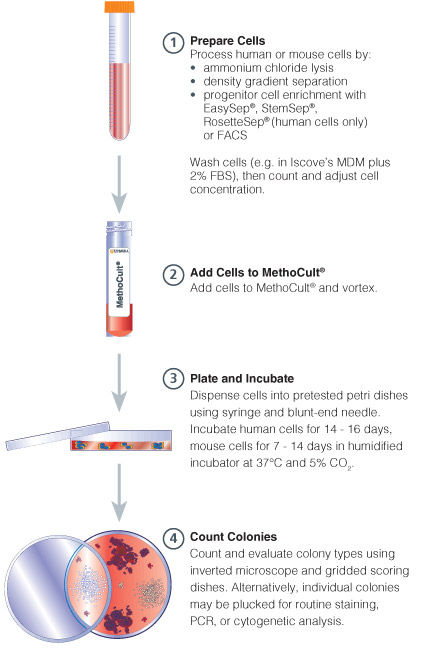
Figure 1. Procedure Summary for Hematopoietic CFU Assays

Figure 2. Examples of Colonies Derived From Mouse Hematopoietic Progenitors
Publications (39)
Nature genetics 2019
Genomic subtyping and therapeutic targeting of acute erythroleukemia.
Abstract
Abstract
Acute erythroid leukemia (AEL) is a high-risk leukemia of poorly understood genetic basis, with controversy regarding diagnosis in the spectrum of myelodysplasia and myeloid leukemia. We compared genomic features of 159 childhood and adult AEL cases with non-AEL myeloid disorders and defined five age-related subgroups with distinct transcriptional profiles: adult, TP53 mutated; NPM1 mutated; KMT2A mutated/rearranged; adult, DDX41 mutated; and pediatric, NUP98 rearranged. Genomic features influenced outcome, with NPM1 mutations and HOXB9 overexpression being associated with a favorable prognosis and TP53, FLT3 or RB1 alterations associated with poor survival. Targetable signaling mutations were present in 45{\%} of cases and included recurrent mutations of ALK and NTRK1, the latter of which drives erythroid leukemogenesis sensitive to TRK inhibition. This genomic landscape of AEL provides the framework for accurate diagnosis and risk stratification of this disease, and the rationale for testing targeted therapies in this high-risk leukemia.
The Journal of biological chemistry 2018 MAY
Gain-of-function mutations in granulocyte colony-stimulating factor receptor (CSF3R) reveal distinct mechanisms of CSF3R activation.
Abstract
Abstract
Granulocyte colony-stimulating factor (G-CSF or CSF3) and its receptor CSF3R regulate granulopoiesis, neutrophil function, and hematopoietic stem cell mobilization. Recent studies have uncovered an oncogenic role of mutations in the CSF3R gene in many hematologic malignancies. To find additional CSF3R mutations that give rise to cell transformation, we performed a cellular transformation assay in which murine interleukin 3 (IL-3)-dependent Ba/F3 cells were transduced with WT CSF3R plasmid and screened for spontaneous growth in the absence of IL-3. Any outgrowth clones were sequenced to identify CSF3R mutations with transformation capacity. We identified several novel mutations and determined that they transform cells via four distinct mechanisms: 1) cysteine- and disulfide bond-mediated dimerization (S581C); 2) polar, noncharged amino acid substitution at the transmembrane helix dimer interface at residue Thr-640; 3) increased internalization by a Glu-524 substitution that mimics a low G-CSF dose; and 4) hydrophobic amino acid substitutions in the membrane-proximal residues Thr-612, Thr-615, and Thr-618. Furthermore, the change in signaling activation was related to an altered CSF3R localization. We also found that CSF3R-induced STAT3 and ERK activations require CSF3R internalization, whereas STAT5 activation occurred at the cell surface. Cumulatively, we have expanded the regions of the CSF3R extracellular and transmembrane domains in which missense mutations exhibit leukemogenic capacity and have further elucidated the mechanistic underpinnings that underlie altered CSF3R expression, dimerization, and signaling activation.
Journal of immunology (Baltimore, Md. : 1950) 2011 MAY
Prevention of bone marrow cell apoptosis and regulation of hematopoiesis by type I IFNs during systemic responses to pneumocystis lung infection.
Abstract
Abstract
We recently demonstrated that lack of type I IFN signaling (IFNAR knockout) in lymphocyte-deficient mice (IFrag(-/-)) results in bone marrow (BM) failure after Pneumocystis lung infection, whereas lymphocyte-deficient mice with intact IFNAR (RAG(-/-)) had normal hematopoiesis. In the current work, we performed studies to define further the mechanisms involved in the induction of BM failure in this system. BM chimera experiments revealed that IFNAR expression was required on BM-derived but not stroma-derived cells to prevent BM failure. Signals elicited after day 7 postinfection appeared critical in determining BM cell fate. We observed caspase-8- and caspase-9-mediated apoptotic cell death, beginning with neutrophils. Death of myeloid precursors was associated with secondary oxidative stress, and decreasing colony-forming activity in BM cell cultures. Treatment with N-acetylcysteine could slow the progression of, but not prevent, BM failure. Type I IFN signaling has previously been shown to expand the neutrophil life span and regulate the expression of some antiapoptotic factors. Quantitative RT-PCR demonstrated reduced mRNA abundance for the antiapoptotic factors BCL-2, IAP2, MCL-1, and others in BM cells from IFrag(-/-) compared with that in BM cells from RAG(-/-) mice at day 7. mRNA and protein for the proapoptotic cytokine TNF-α was increased, whereas mRNA for the growth factors G-CSF and GM-CSF was reduced. In vivo anti-TNF-α treatment improved precursor cell survival and activity in culture. Thus, we propose that lack of type I IFN signaling results in decreased resistance to inflammation-induced proapoptotic stressors and impaired replenishment by precursors after systemic responses to Pneumocystis lung infection. Our finding may have implications in understanding mechanisms underlying regenerative BM depression/failure during complex immune deficiencies such as AIDS.
Blood 2011 JUN
DOT1L, the H3K79 methyltransferase, is required for MLL-AF9-mediated leukemogenesis.
Abstract
Abstract
Chromosomal translocations of the mixed lineage leukemia (MLL) gene are a common cause of acute leukemias. The oncogenic function of MLL fusion proteins is, in part, mediated through aberrant activation of Hoxa genes and Meis1, among others. Here we demonstrate using a tamoxifen-inducible Cre-mediated loss of function mouse model that DOT1L, an H3K79 methyltransferase, is required for both initiation and maintenance of MLL-AF9-induced leukemogenesis in vitro and in vivo. Through gene expression and chromatin immunoprecipitation analysis we demonstrate that mistargeting of DOT1L, subsequent H3K79 methylation, and up-regulation of Hoxa and Meis1 genes underlie the molecular mechanism of how DOT1L contributes to MLL-AF9-mediated leukemogenesis. Our study not only provides the first in vivo evidence for the function of DOT1L in leukemia, but also reveals the molecular mechanism for DOT1L in MLL-AF9 mediated leukemia. Thus, DOT1L may serve as a potential therapeutic target for the treatment of leukemia caused by MLL translocations.
Experimental biology and medicine (Maywood, N.J.) 2011 JUN
Protective effect of dammarane sapogenins against chemotherapy-induced myelosuppression in mice.
Abstract
Abstract
Chemotherapy is the most common way to treat malignancies, but myelosuppression, one of its common side-effects, is a formidable problem. The present study described the protective role of dammarane sapogenins (DS), an active fraction from oriental ginseng, on myelosuppression induced by cyclophosphamide (CP) in mice. DS was orally administered at different dosages (37.5, 75, and 150 mg/kg) for 10 d after CP administration (200 mg/kg intraperitoneally). The results showed that DS increased the number of white blood cells (WBC) on day 3 and day 7 (P textless 0.05), such that WBC levels were increased by 105.7 ± 29.5% at 75 mg/kg of DS on day 3 (P textless 0.05, compared with the CP group). Similar results were observed in red blood cells and platelets in DS-treated groups. The colony-forming assay demonstrated that the depressed numbers of CFU-GM (colony-forming unit-granulocyte and macrophage), CFU-E (colony-forming unit-erythroid), BFU-E (burst-forming unit-erythroid), CFU-Meg (colony-forming unit-megakaryocyte) and CFU-GEMM (colony-forming unit-granulocyte, -erythrocyte, -monocyte and -megakaryocyte) induced by CP were significantly reversed after DS treatment. Moreover, the ameliorative effect of DS on myelosuppression was also observed in the femur by hematoxylin/eosin staining. In DS-treated groups, ConA-induced splenocyte proliferation was enhanced significantly at all the doses (37.5, 75, 150 mg/kg) on day 3 at the rate of 50.3 ± 8.0%, 77.6 ± 8.5% and 44.5 ± 8.4%, respectively, while lipopolysaccharide-induced proliferation was increased mainly on day 7 (P textless 0.01), with an increased rate of 39.8 ± 5.6%, 34.9 ± 6.6% and 38.3 ± 7.3%, respectively. The thymus index was also markedly increased by 70.4% and 36.6% at 75 mg/kg on days 3 and 7, respectively, as compared with the CP group. In summary, DS has a protective function against CP-induced myelosuppression. Its mechanism might be related to stimulating hematopoiesis recovery, as well as enhancing the immunological function.
Blood 2011 JUN
3'UTR-truncated Hmga2 cDNA causes MPN-like hematopoiesis by conferring a clonal growth advantage at the level of HSC in mice.
Abstract
Abstract
Overexpression of high mobility group AT-hook 2 (HMGA2) is found in a number of benign and malignant tumors, including the clonal PIGA(-) cells in 2 cases of paroxysmal nocturnal hemoglobinuria (PNH) and some myeloproliferative neoplasms (MPNs), and recently in hematopoietic cell clones resulting from gene therapy procedures. In nearly all these cases overexpression is because of deletions or translocations that remove the 3' untranslated region (UTR) which contains binding sites for the regulatory micro RNA let-7. We were therefore interested in the effect of HMGA2 overexpression in hematopoietic tissues in transgenic mice (ΔHmga2 mice) carrying a 3'UTR-truncated Hmga2 cDNA. ΔHmga2 mice expressed increased levels of HMGA2 protein in various tissues including hematopoietic cells and showed proliferative hematopoiesis with increased numbers in all lineages of peripheral blood cells, hypercellular bone marrow (BM), splenomegaly with extramedullary erythropoiesis and erythropoietin-independent erythroid colony formation. ΔHmga2-derived BM cells had a growth advantage over wild-type cells in competitive repopulation and serial transplantation experiments. Thus overexpression of HMGA2 leads to proliferative hematopoiesis with clonal expansion at the stem cell and progenitor levels and may account for the clonal expansion in PNH and MPNs and in gene therapy patients after vector insertion disrupts the HMGA2 locus.