MesenCult™ Proliferation Kit (Human)
Medium for detection of CFU-F and expansion of human mesenchymal stem cells
概要
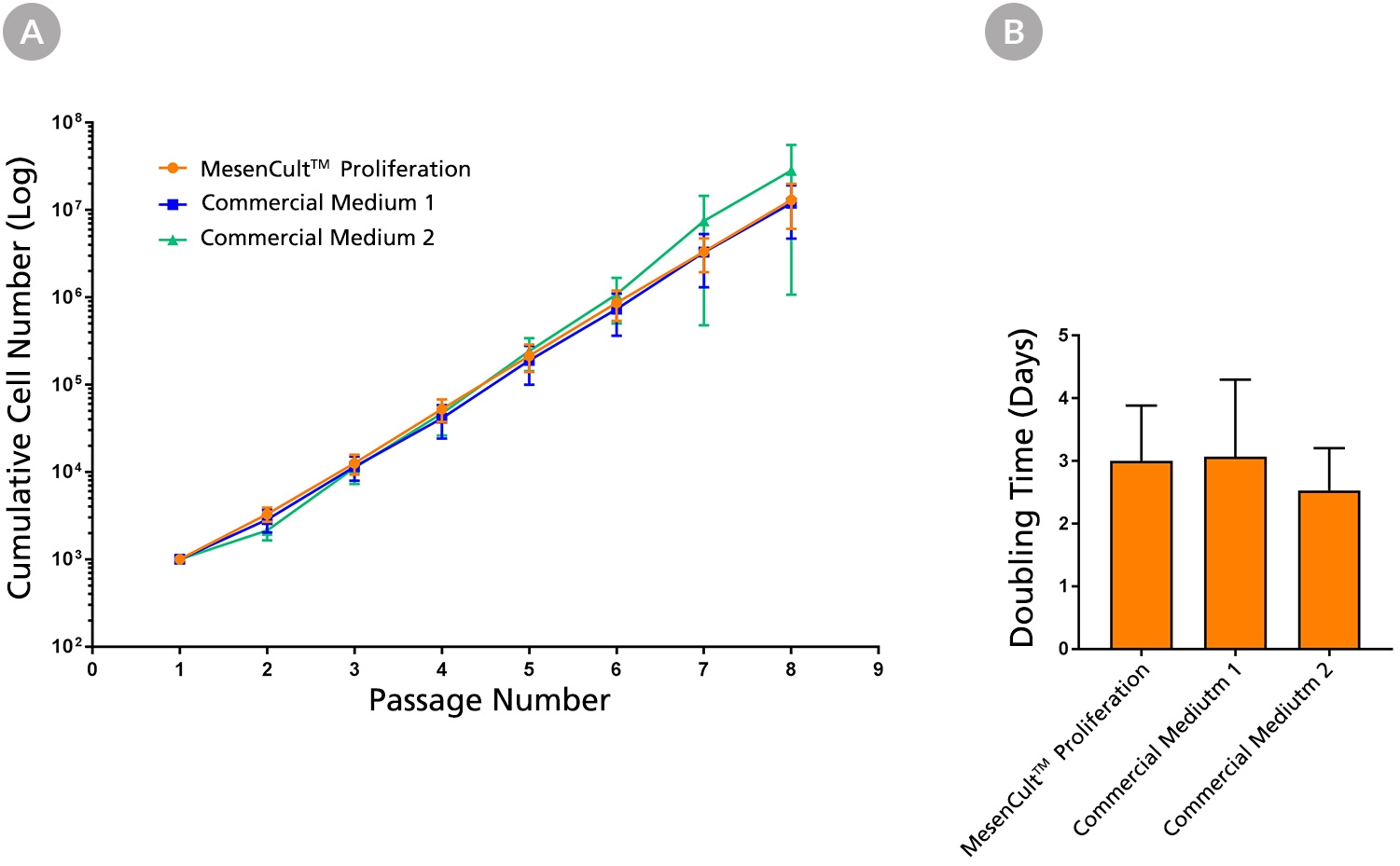
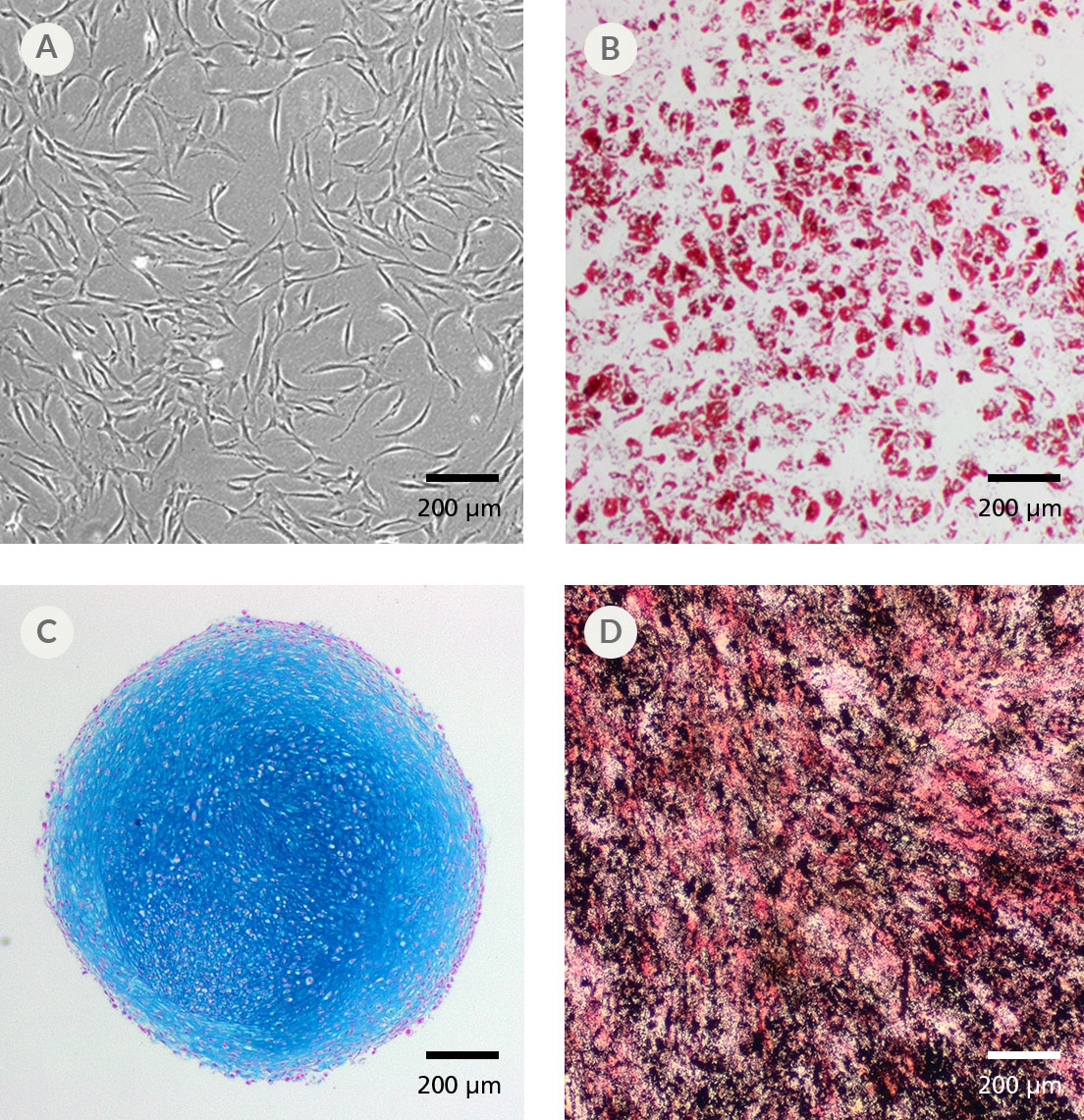
MesenCult™ Proliferation Kit (Human) is a standardized, serum-containing medium for the culture of human mesenchymal stem cells (MSCs). MesenCult™ Proliferation Kit (Human) is optimized for the expansion of human MSCs in vitro as well as for the detection and enumeration of colony-forming unit - fibroblasts (CFU-F). MesenCult™ Proliferation Kit (Human) includes MesenCult™ MSC Basal Medium (Human; 450 mL) and MesenCult™ MSC Stimulatory Supplement (Human; 50 mL).
- MesenCult™ MSC Basal Medium (Human), 450 mL (Catalog #05401)
- MesenCult™ MSC Stimulatory Supplement (Human), 50 mL (Catalog #05402)
Mesenchymal Stem and Progenitor Cells
Cell Culture, Colony Assay, Expansion
数据及文献
Publications (39)
International journal of molecular sciences 2020 mar
Thymosin $\beta$4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model.
J.-H. Kim et al.
Abstract
Thymosin $\beta$4 (T$\beta$4) is a G-actin sequestering protein that contributes to diverse cellular activities, such as migration and angiogenesis. In this study, the beneficial effects of combined cell therapy with T$\beta$4 and human adipose-derived stem cells (hASCs) in a mouse ischemic hindlimb model were investigated. We observed that exogenous treatment with T$\beta$4 enhanced endogenous TMSB4X mRNA expression and promoted morphological changes (increased cell length) in hASCs. Interestingly, T$\beta$4 induced the active state of hASCs by up-regulating intracellular signaling pathways including the PI3K/AKT/mTOR and MAPK/ERK pathways. Treatment with T$\beta$4 significantly increased cell migration and sprouting from microbeads. Moreover, additional treatment with T$\beta$4 promoted the endothelial differentiation potential of hASCs by up-regulating various angiogenic genes. To evaluate the in vivo effects of the T$\beta$4-hASCs combination on vessel recruitment, dorsal window chambers were transplanted, and the co-treated mice were found to have a significantly increased number of microvessel branches. Transplantation of hASCs in combination with T$\beta$4 was found to improve blood flow and attenuate limb or foot loss post-ischemia compared to transplantation with hASCs alone. Taken together, the therapeutic application of hASCs combined with T$\beta$4 could be effective in enhancing endothelial differentiation and vascularization for treating hindlimb ischemia.
Cell biology international 2012 JUL
New approach to isolate mesenchymal stem cell (MSC) from human umbilical cord blood.
Hussain I et al.
Abstract
HUCB (human umbilical cord blood) has been frequently used in clinical allogeneic HSC (haemopoietic stem cell) transplant. However, HUCB is poorly recognized as a rich source of MSC (mesenchymal stem cell). The aim of this study has been to establish a new method for isolating large number of MSC from HUCB to recognize it as a good source of MSC. HUCB samples were collected from women following their elective caesarean section. The new method (Clot Spot method) was carried out by explanting HUCB samples in mesencult complete medium and maintained in 37°C, in a 5% CO2 and air incubator. MSC presence was established by quantitative and qualitative immunophenotyping of cells and using FITC attached to MSC phenotypic markers (CD29, CD73, CD44 and CD105). Haematopoietic antibodies (CD34 and CD45) were used as negative control. MSC differentiation was examined in neurogenic and adipogenic media. Immunocytochemistry was carried out for the embryonic markers: SOX2 (sex determining region Y-box 2), OLIG-4 (oligodendrocyte-4) and FABP-4 (fatty acid binding protein-4). The new method was compared with the conventional Rosset Sep method. MSC cultures using the Clot Spot method showed 3-fold increase in proliferation rate compared with conventional method. Also, the cells showed high expression of MSC markers CD29, CD73, CD44 and CD105, but lacked the expression of specific HSC markers (CD34 and CD45). The isolated MSC showed some differentiation by expressing the neurogenic (SOX2 and Olig4) and adipogenic (FABP-4) markers respectively. In conclusion, HUCB is a good source of MSC using this new technique.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011 OCT
Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro.
Mendelson A et al.
Abstract
Cell transplantation has been well explored for cartilage regeneration. We recently showed that the entire articular surface of a synovial joint can regenerate by endogenous cell homing and without cell transplantation. However, the sources of endogenous cells that regenerate articular cartilage remain elusive. Here, we studied whether cytokines not only chemotactically recruit adipose stem cells (ASCs), mesenchymal stem cells (MSCs), and synovium stem cells (SSCs) but also induce chondrogenesis of the recruited cells. Recombinant human transforming growth factor-β3 (TGF-β3; 100 ng) and/or recombinant human stromal derived factor-1β (SDF-1β; 100 ng) was control released into an acellular collagen sponge cube with underlying ASCs, MSCs, or SSCs in monolayer culture. Although all cell types randomly migrated into the acellular collagen sponge cube, TGF-β3 and/or SDF-1β recruited significantly more cells than the cytokine-free control group. In 6 wk, TGF-β3 alone recruited substantial numbers of ASCs (558±65) and MSCs (302±52), whereas codelivery of TGF-β3 and SDF-1β was particularly chemotactic to SSCs (400±120). Proliferation of the recruited cells accounted for some, but far from all, of the observed cellularity. TGF-β3 and SDF-1β codelivery induced significantly higher aggrecan gene expression than the cytokine-free group for ASCs, MSCs, and SSCs. Type II collagen gene expression was also significantly higher for ASCs and SSCs by SDF-1 and TGF-β3 codelivery. Remarkably, the expression of aggrecan and type II collagen was detected among all cell types. Thus, homing of multiple stem/progenitor cell populations may potentially serve as an alternative or adjunctive approach to cell transplantation for cartilage regeneration.
Experimental cell research 2011 NOV
Focal adhesion protein abnormalities in myelodysplastic mesenchymal stromal cells.
Aanei CM et al.
Abstract
Direct cell-cell contact between haematopoietic progenitor cells (HPCs) and their cellular microenvironment is essential to maintain 'stemness'. In cancer biology, focal adhesion (FA) proteins are involved in survival signal transduction in a wide variety of human tumours. To define the role of FA proteins in the haematopoietic microenvironment of myelodysplastic syndromes (MDS), CD73-positive mesenchymal stromal cells (MSCs) were immunostained for paxillin, pFAK [Y(397)], and HSP90α/β and p130CAS, and analysed for reactivity, intensity and cellular localisation. Immunofluorescence microscopy allowed us to identify qualitative and quantitative differences, and subcellular localisation analysis revealed that in pathological MSCs, paxillin, pFAK [Y(397)], and HSP90α/β formed nuclear molecular complexes. Increased expression of paxillin, pFAK [Y(397)], and HSP90α/β and enhanced nuclear co-localisation of these proteins correlated with a consistent proliferative advantage in MSCs from patients with refractory anaemia with excess blasts (RAEB) and negatively impacted clonogenicity of HPCs. These results suggest that signalling via FA proteins could be implicated in HPC-MSC interactions. Further, because FAK is an HSP90α/β client protein, these results suggest the utility of HSP90α/β inhibition as a target for adjuvant therapy for myelodysplasia.
Blood 2011 AUG
Adult human circulating CD34 cells can differentiate into hematopoietic and endothelial cells.
Ciraci E et al.
Abstract
A precise identification of adult human hemangioblast is still lacking. To identify circulating precursors having the developmental potential of the hemangioblast, we established a new ex vivo long-term culture model supporting the differentiation of both hematopoietic and endothelial cell lineages. We identified from peripheral blood a population lacking the expression of CD34, lineage markers, CD45 and CD133 (CD34⁻Lin⁻CD45⁻CD133⁻ cells), endowed with the ability to differentiate after a 6-week culture into both hematopoietic and endothelial lineages. The bilineage potential of CD34⁻Lin⁻CD45⁻CD133⁻ cells was determined at the single-cell level in vitro and was confirmed by transplantation into NOD/SCID mice. In vivo, CD34⁻Lin⁻CD45⁻CD133⁻ cells showed the ability to reconstitute hematopoietic tissue and to generate functional endothelial cells that contribute to new vessel formation during tumor angiogenesis. Molecular characterization of CD34⁻Lin⁻D45⁻CD133⁻ cells unveiled a stem cell profile compatible with both hematopoietic and endothelial potentials, characterized by the expression of c-Kit and CXCR4 as well as EphB4, EphB2, and ephrinB2. Further molecular and functional characterization of CD34⁻Lin⁻CD45⁻CD133⁻ cells will help dissect their physiologic role in blood and blood vessel maintenance and repair in adult life.
Progress in biophysics and molecular biology 2010 SEP
Unique biomechanical interactions between myeloma cells and bone marrow stroma cells.
Feng Y et al.
Abstract
We observed that BMSCs (bone marrow stromal cells) from myeloma patients (myeloma BMSCs) were significantly stiffer than control BMSCs using a cytocompression device. The stiffness of myeloma BMSCs and control BMSCs was further increased upon priming by myeloma cells. Additionally, myeloma cells became stiffer when primed by myeloma BMSCs. The focal adhesion kinase activity of myeloma cells was increased when cells were on stiffer collagen gels and on myeloma BMSCs. This change in myeloma stiffness is associated with increased colony formation of myeloma cells and FAK activation when co-cultured with stiffer myeloma BMSCs or stiffer collagen. Additionally, stem cells of RPMI8226 cells became stiffer after priming by myeloma BMSCs, with concomitant increases of stem cell colony formation. These results suggest the presence of a mechanotransduction loop between myeloma cells and myeloma BMSCs to increase the stiffness of both types of cells via FAK activation. The increase of stiffness may in turn support the growth of myeloma cells and myeloma stem cells.
View All Publications