MesenCult™ Osteogenic Stimulatory Kit (Mouse)
Complete medium for differentiating mouse mesenchymal stem cells and embryonic fibroblasts into osteoblasts
概要
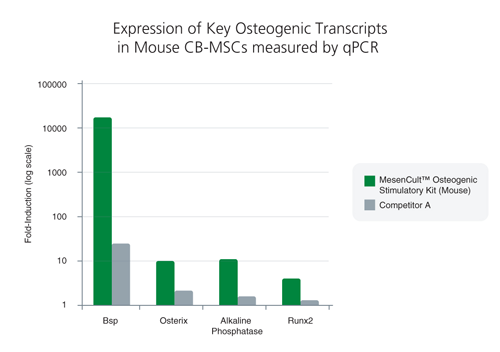
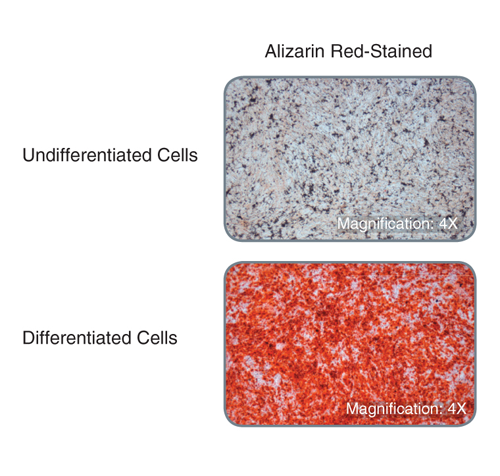
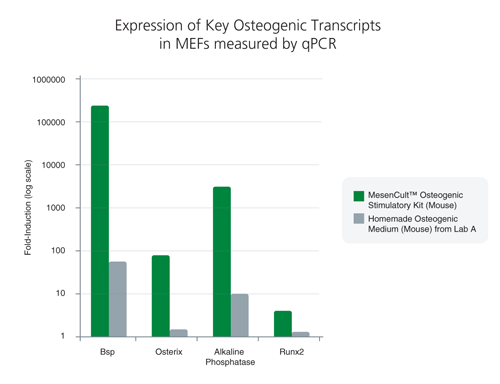
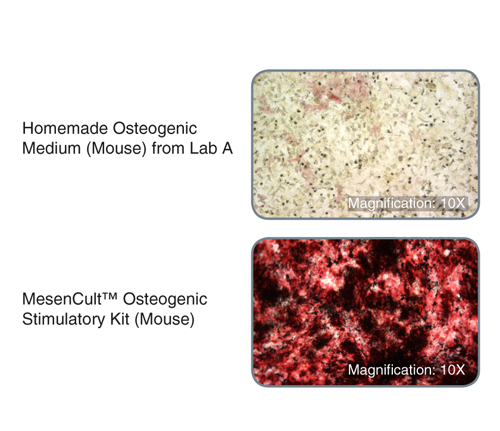
MesenCult™ Osteogenic Stimulatory Kit (Mouse) is specifically formulated for the in vitro differentiation of mouse mesenchymal stem and progenitor cells (MSCs) from compact bone and bone marrow, and mouse embryonic fibroblasts (MEFs) into osteoblasts. This kit induces strong osteogenesis of mouse MSCs and MEFs, as evidenced by qPCR analysis of key transcripts involved in bone differentiation and maturation, and Alizarin Red staining. This kit is recommended for characterizing MSCs and MEFs and studying bone development.
This kit induces robust osteogenesis of culture-expanded mouse mesenchymal stem and progenitor cells and embryonic fibroblasts as evidenced by superior expression of key osteogenic transcripts involved in bone differentiation and maturation, and Alizarin Red staining. See Data and Publications tab.
• MesenCult™ MSC Basal Medium (Mouse), 200 mL
• MesenCult™ Osteogenic Stimulatory Supplement (Mouse), 50 mL
Mesenchymal Stem and Progenitor Cells, Mouse Embryonic Fibroblasts, Osteoblasts
Cell Culture, Differentiation
数据及文献
Publications (6)
Stem cells translational medicine 2020 jan
Radiation mitigation of the intestinal acute radiation injury in mice by 1-[(4-nitrophenyl)sulfonyl]-4-phenylpiperazine.
S. Duhachek-Muggy et al.
Abstract
The objective of the study was to identify the mechanism of action for a radiation mitigator of the gastrointestinal (GI) acute radiation syndrome (ARS), identified in an unbiased high-throughput screen. We used mice irradiated with a lethal dose of radiation and treated with daily injections of the radiation mitigator 1-[(4-nitrophenyl)sulfonyl]-4-phenylpiperazine to study its effects on key pathways involved in intestinal stem cell (ISC) maintenance. RNASeq, quantitative reverse transcriptase-polymerase chain reaction, and immunohistochemistry were performed to identify pathways engaged after drug treatment. Target validation was performed with competition assays, reporter cells, and in silico docking. 1-[(4-Nitrophenyl)sulfonyl]-4-phenylpiperazine activates Hedgehog signaling by binding to the transmembrane domain of Smoothened, thereby expanding the ISC pool, increasing the number of regenerating crypts and preventing the GI-ARS. We conclude that Smoothened is a target for radiation mitigation in the small intestine that could be explored for use in radiation accidents as well as to mitigate normal tissue toxicity during and after radiotherapy of the abdomen.
Cell stem cell 2019 nov
The RNA Helicase DDX6 Controls Cellular Plasticity by Modulating P-Body Homeostasis.
B. Di Stefano et al.
Abstract
Post-transcriptional mechanisms have the potential to influence complex changes in gene expression, yet their role in cell fate transitions remains largely unexplored. Here, we show that suppression of the RNA helicase DDX6 endows human and mouse primed embryonic stem cells (ESCs) with a differentiation-resistant, hyper-pluripotent" state which readily reprograms to a naive state resembling the preimplantation embryo. We further demonstrate that DDX6 plays a key role in adult progenitors where it controls the balance between self-renewal and differentiation in a context-dependent manner. Mechanistically DDX6 mediates the translational suppression of target mRNAs in P-bodies. Upon loss of DDX6 activity P-bodies dissolve and release mRNAs encoding fate-instructive transcription and chromatin factors that re-enter the ribosome pool. Increased translation of these targets impacts cell fate by rewiring the enhancer heterochromatin and DNA methylation landscapes of undifferentiated cell types. Collectively our data establish a link between P-body homeostasis chromatin organization and stem cell potency."
International journal of molecular sciences 2019 nov
Quercetin Exposure Suppresses the Inflammatory Pathway in Intestinal Organoids from Winnie Mice.
M. Dicarlo et al.
Abstract
Inflammatory bowel diseases (IBDs) are chronic and relapsing immune disorders that result, or possibly originate, from epithelial barrier defects. Intestinal organoids are a new reliable tool to investigate epithelial response in models of chronic inflammation. We produced organoids from the ulcerative colitis murine model Winnie to explore if the chronic inflammatory features observed in the parental intestine were preserved by the organoids. Furthermore, we investigated if quercetin administration to in vitro cultured organoids could suppress LPS-induced inflammation in wild-type organoids (WT-organoids) and spontaneous inflammation in ulcerative colitis organoids (UC-organoids). Our data demonstrate that small intestinal organoids obtained from Winnie mice retain the chronic intestinal inflammatory features characteristic of the parental tissue. Quercetin administration was able to suppress inflammation both in UC-organoids and in LPS-treated WT-organoids. Altogether, our data demonstrate that UC-organoids are a reliable experimental system for investigating chronic intestinal inflammation and pharmacological responses.
Journal of biomedical materials research. Part A 2019 jul
Aligned fibrous decellularized cell derived matrices for mesenchymal stem cell amplification.
M. Ventre et al.
Abstract
Biochemical and biophysical stimuli of stem cell niches finely regulate the self-renewal/differentiation equilibrium. Replicating this in vitro is technically challenging, making the control of stem cell functions difficult. Cell derived matrices capture certain aspect of niches that influence fate decisions. Here, aligned fibrous matrices synthesized by MC3T3 cells were produced and the role of matrix orientation and stiffness on the maintenance of stem cell characteristics and adipo- or osteo-genic differentiation of murine mesenchymal stem cells (mMSCs) was investigated. Decellularized matrices promoted mMSC proliferation. Fibrillar alignment and matrix stiffness work in concert in defining cell fate. Soft matrices preserve stemness, whereas stiff ones, in presence of biochemical supplements, promptly induce differentiation. Matrix alignment impacts the homogeneity of the cell population, that is, soft aligned matrices ameliorate the spontaneous adipogenic differentiation, whereas stiff aligned matrices reduce cross-differentiation. We infer that mechanical signaling is a dominant factor in mMSC fate decision and the matrix alignment contributes to produce a more homogeneous environment, which results in a uniform response of cells to biophysical environment. Matrix thus produced can be obtained in vitro in a facile and consistent manner and can be used for homogeneous stem cell amplification or for mechanotransduction-related studies.
Science (New York, N.Y.) 2019
Identification of a mesenchymal progenitor cell hierarchy in adipose tissue.
D. Merrick et al.
Abstract
Metabolic health depends on the capacity of adipose tissue progenitor cells to undergo de novo adipogenesis. The cellular hierarchy and mechanisms governing adipocyte progenitor differentiation are incompletely understood. Through single-cell RNA sequence analyses, we show that the lineage hierarchy of adipocyte progenitors consists of distinct mesenchymal cell types that are present in both mouse and human adipose tissues. Cells marked by dipeptidyl peptidase-4 (DPP4)/CD26 expression are highly proliferative, multipotent progenitors. During the development of subcutaneous adipose tissue in mice, these progenitor cells give rise to intercellular adhesion molecule-1 (ICAM1)/CD54-expressing (CD54+) committed preadipocytes and a related adipogenic cell population marked by Clec11a and F3/CD142 expression. Transforming growth factor-beta maintains DPP4+ cell identity and inhibits adipogenic commitment of DPP4+ and CD142+ cells. Notably, DPP4+ progenitors reside in the reticular interstitium, a recently appreciated fluid-filled space within and between tissues, including adipose depots.
Frontiers in immunology 2018
Hookworm Secreted Extracellular Vesicles Interact With Host Cells and Prevent Inducible Colitis in Mice.
R. M. Eichenberger et al.
Abstract
Gastrointestinal (GI) parasites, hookworms in particular, have evolved to cause minimal harm to their hosts, allowing them to establish chronic infections. This is mediated by creating an immunoregulatory environment. Indeed, hookworms are such potent suppressors of inflammation that they have been used in clinical trials to treat inflammatory bowel diseases (IBD) and celiac disease. Since the recent description of helminths (worms) secreting extracellular vesicles (EVs), exosome-like EVs from different helminths have been characterized and their salient roles in parasite-host interactions have been highlighted. Here, we analyze EVs from the rodent parasite Nippostrongylus brasiliensis, which has been used as a model for human hookworm infection. N. brasiliensis EVs (Nb-EVs) are actively internalized by mouse gut organoids, indicating a role in driving parasitism. We used proteomics and RNA-Seq to profile the molecular composition of Nb-EVs. We identified 81 proteins, including proteins frequently present in exosomes (like tetraspanin, enolase, 14-3-3 protein, and heat shock proteins), and 27 sperm-coating protein-like extracellular proteins. RNA-Seq analysis revealed 52 miRNA species, many of which putatively map to mouse genes involved in regulation of inflammation. To determine whether GI nematode EVs had immunomodulatory properties, we assessed their potential to suppress GI inflammation in a mouse model of inducible chemical colitis. EVs from N. brasiliensis but not those from the whipworm Trichuris muris or control vesicles from grapes protected against colitic inflammation in the gut of mice that received a single intraperitoneal injection of EVs. Key cytokines associated with colitic pathology (IL-6, IL-1$\beta$, IFN$\gamma$, and IL-17a) were significantly suppressed in colon tissues from EV-treated mice. By contrast, high levels of the anti-inflammatory cytokine IL-10 were detected in Nb-EV-treated mice. Proteins and miRNAs contained within helminth EVs hold great potential application in development of drugs to treat helminth infections as well as chronic non-infectious diseases resulting from a dysregulated immune system, such as IBD.
View All Publications