MesenCult™ Osteogenic Differentiation Kit (Human)
For the in vitro differentiation of human MSCs into osteoblasts
概要
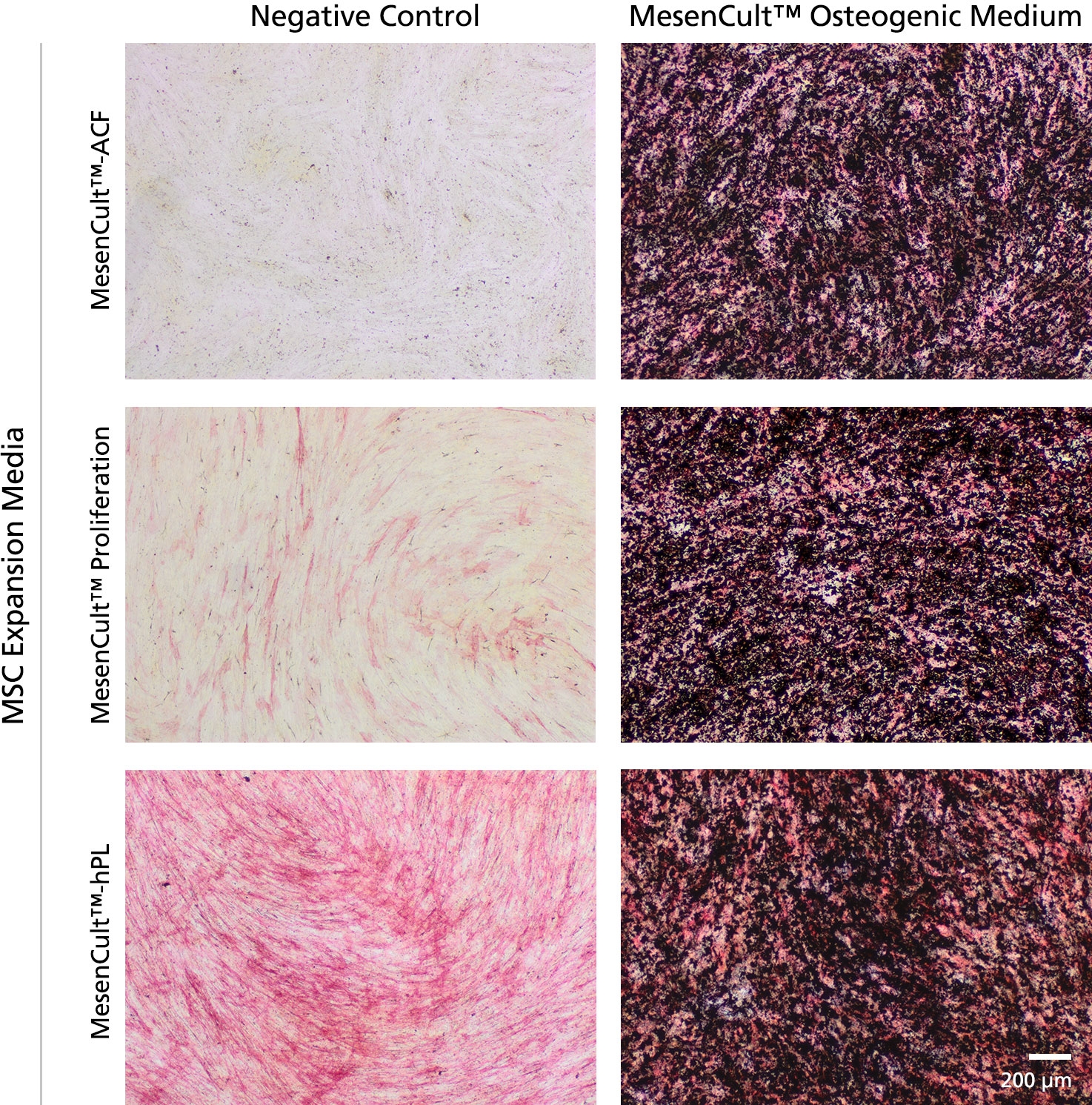
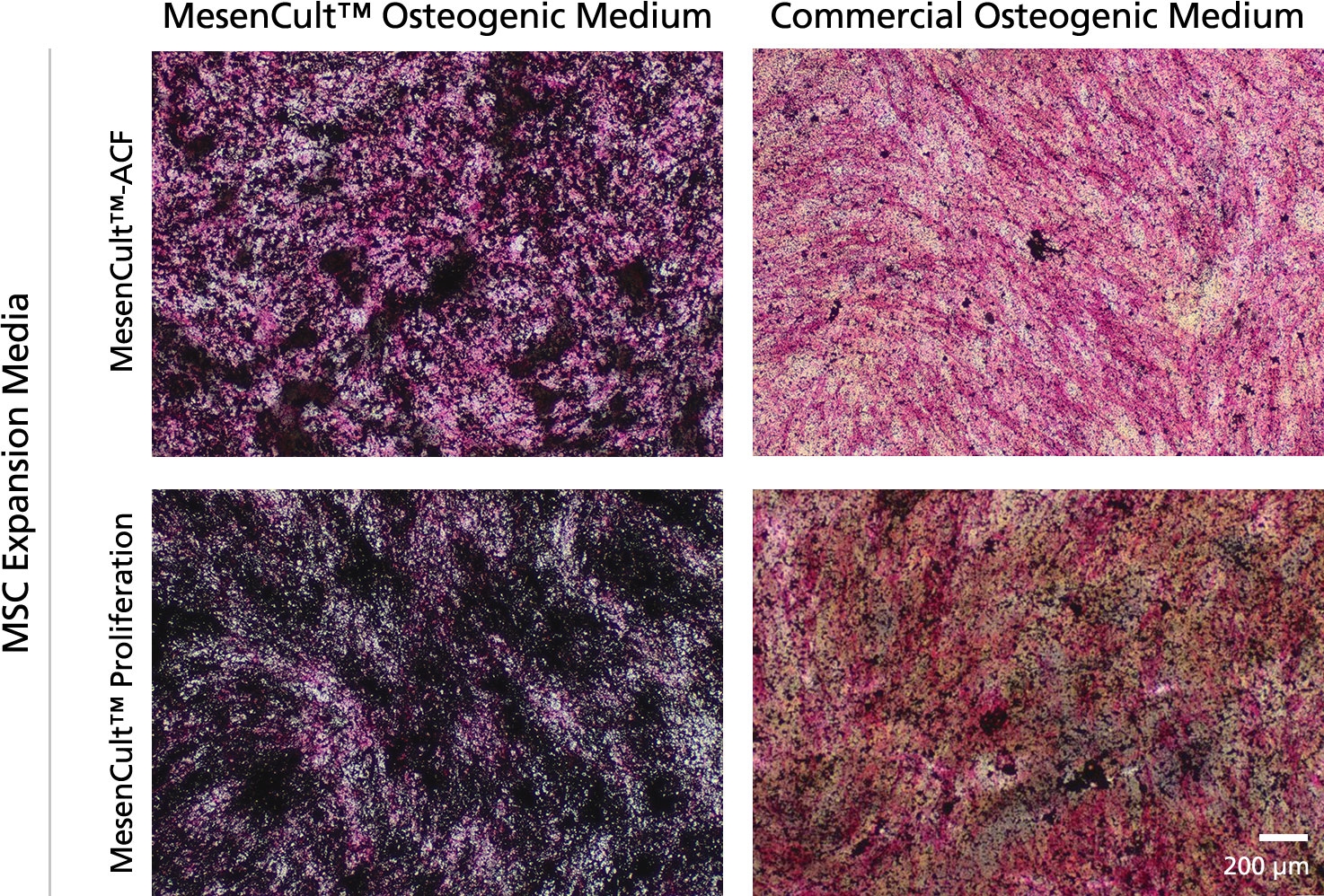
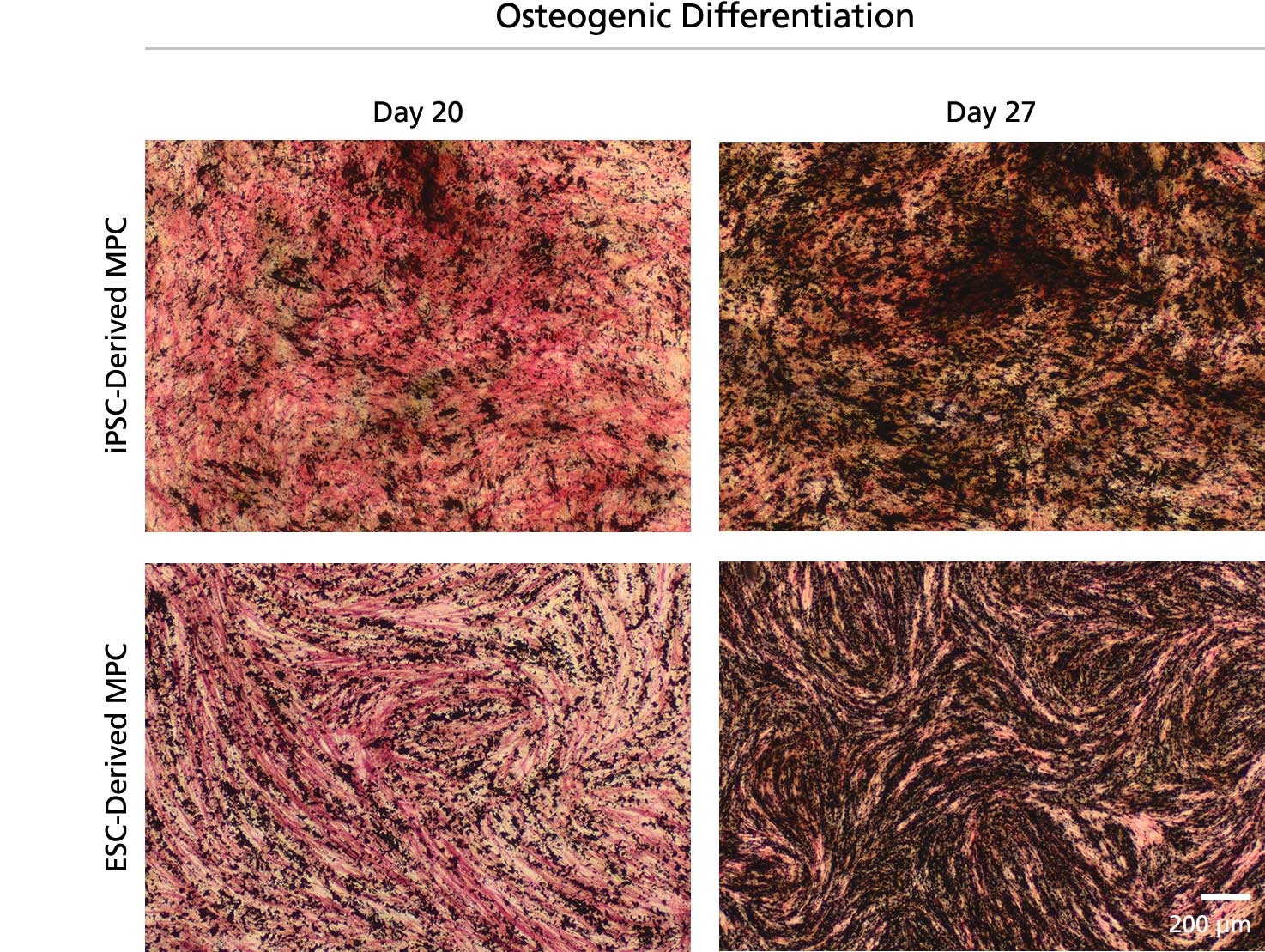
MesenCult™ Osteogenic Differentiation Kit (Human) is specifically formulated for the in vitro differentiation of human mesenchymal stem and progenitor cells (MSCs) into cells of the osteogenic lineage. This kit is suitable for the differentiation of human bone marrow (BM)-derived MSCs previously culture-expanded in serum-containing medium (e.g. MesenCult™ Proliferation Kit [Human; Catalog #05411] or MesenCult™-hPL [Human; Catalog #05439]) or animal component-free MesenCult™-ACF Plus Medium [Catalog #05445]).
• Compatible with human MSCs previously culture-expanded in MesenCult™ expansion media.
• Available in an easy-to-use two-component format.
• Rigorous raw material screening and quality control minimize lot-to-lot variability.
- MesenCult™ Osteogenic Differentiation Basal Medium (Human), 200 mL
- MesenCult™ Osteogenic Differentiation 5X Supplement (Human), 50 mL
Mesenchymal Stem and Progenitor Cells, Osteoblasts
Cell Culture, Differentiation
数据及文献
Publications (9)
Science advances 2020 may
In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion.
L. Wang et al.
Abstract
Increasing occurrence of moderate to severe intrauterine adhesion (IUA) is seriously affecting the quality of human life. The aim of the study was to establish IUA models in nonhuman primates and to explore the dual repair effects of human umbilical cord-derived mesenchymal stem cells (huMSCs) loaded on autocrosslinked hyaluronic acid gel (HA-GEL) on endometrial damage and adhesion. Here, we recorded the menstrual cycle data in detail with uterine cavities observed and endometrial tissues detected after intervention, and the thicker endometria, decreased amount of fibrotic formation, increased number of endometrium glands, etc., suggested that both HA-GEL and huMSC/HA-GEL complexes could partially repair IUA caused by mechanical injury, but huMSC/HA-GEL complex transplantation had notable dual repair effects: a reliable antiadhesion property and the promotion of endometrial regeneration.
Polymers 2020 jun
Altered Surface Hydrophilicity on Copolymer Scaffolds Stimulate the Osteogenic Differentiation of Human Mesenchymal Stem Cells.
Z. Xing et al.
Abstract
BACKGROUND Recent studies have suggested that both poly(l-lactide-co-1,5-dioxepan-2-one) (or poly(LLA-co-DXO)) and poly(l-lactide-co-$\epsilon$-caprolactone) (or poly(LLA-co-CL)) porous scaffolds are good candidates for use as biodegradable scaffold materials in the field of tissue engineering; meanwhile, their surface properties, such as hydrophilicity, need to be further improved. METHODS We applied several different concentrations of the surfactant Tween 80 to tune the hydrophilicity of both materials. Moreover, the modification was applied not only in the form of solid scaffold as a film but also a porous scaffold. To investigate the potential application for tissue engineering, human bone marrow mesenchymal stem cells (hMSCs) were chosen to test the effect of hydrophilicity on cell attachment, proliferation, and differentiation. First, the cellular cytotoxicity of the extracted medium from modified scaffolds was investigated on HaCaT cells. Then, hMSCs were seeded on the scaffolds or films to evaluate cell attachment, proliferation, and osteogenic differentiation. The results indicated a significant increasing of wettability with the addition of Tween 80, and the hMSCs showed delayed attachment and spreading. PCR results indicated that the differentiation of hMSCs was stimulated, and several osteogenesis related genes were up-regulated in the 3{\%} Tween 80 group. Poly(LLA-co-CL) with 3{\%} Tween 80 showed an increased messenger Ribonucleic acid (mRNA) level of late-stage markers such as osteocalcin (OC) and key transcription factor as runt related gene 2 (Runx2). CONCLUSION A high hydrophilic scaffold may speed up the osteogenic differentiation for bone tissue engineering.
Stem cells and development 2020 jul
Human Umbilical Cord Mesenchymal Stem Cells Attenuate Abdominal Aortic Aneurysm Progression in Sprague-Dawley Rats: Implication of Vascular Smooth Muscle Cell Phenotypic Modulation.
H. Wen et al.
Abstract
Abdominal aortic aneurysm (AAA) is life-threatening, for which efficient nonsurgical treatment strategy has not been available so far. Several previous studies investigating the therapeutic effect of mesenchymal stem cells (MSCs) in AAA indicated that MSCs could inhibit aneurysmal inflammatory responses and extracellular matrix destruction, and suppress aneurysm occurrence and expansion. Vascular smooth muscle cell (VSMC) phenotypic plasticity is reported to be predisposed in AAA initiation and progression. However, little is known about the effect of MSCs on VSMC phenotypic modulation in AAA. In this study, we investigate the therapeutic efficacy of umbilical cord mesenchymal stem cells (UC-MSCs) in elastase-induced AAA model and evaluate the effect of UC-MSC on VSMC phenotypic regulation. We demonstrate that the intravenous injection of UC-MSC attenuates elastase-induced aneurysmal expansion, reduces elastin degradation and fragmentation, inhibits MMPs and TNF-$\alpha$ expression, and preserves and/or restores VSMC contractile phenotype in AAA. Taken together, these results highlight the therapeutic and VSMC phenotypic modulation effects of UC-MSC in AAA progression, which further indicates the potential of applying UC-MSC as an alternative treatment candidate for AAA.
Stem cell research {\&} therapy 2020 jan
Scaffold vascularization method using an adipose-derived stem cell (ASC)-seeded scaffold prefabricated with a flow-through pedicle.
T. D\cebski et al.
Abstract
BACKGROUND Vascularization is important for the clinical application of tissue engineered products. Both adipose-derived stem cells (ASCs) and surgical prefabrication can be used to induce angiogenesis in scaffolds. Our aim was to compare the angiogenic potential of ASC-seeded scaffolds combined with scaffold prefabrication with that of non-seeded, non-prefabricated scaffolds. METHODS For prefabrication, functional blood vessels were introduced into the scaffold using a flow-through pedicle system. ASCs were isolated from rat fat deposits. Three-dimensional-printed cylindrical poly-$\epsilon$-caprolactone scaffolds were fabricated by fused deposition modelling. Three groups, each containing six rats, were investigated by using non-seeded, ASC-seeded, and osteogenic induced ASC-seeded scaffolds. In each group, one rat was implanted with two scaffolds in the inguinal region. On the right side, a scaffold was implanted subcutaneously around the inferior epigastric vessels (classic prefabrication group). On the left side, the inferior epigastric vessels were placed inside the prefabricated scaffold in the flow-through pedicle system (flow-through prefabrication group). The vessel density and vascular architecture were examined histopathologically and by $\mu$CT imaging, respectively, at 2 months after implantation. RESULTS The mean vessel densities were 10- and 5-fold higher in the ASC-seeded and osteogenic induced ASC-seeded scaffolds with flow-through prefabrication, respectively, than in the non-seeded classic prefabricated group (p {\textless} 0.001). $\mu$CT imaging revealed functional vessels within the scaffold. CONCLUSION ASC-seeded scaffolds with prefabrication showed significantly improved scaffold vasculogenesis and could be useful for application to tissue engineering products in the clinical settings.
Medicinski glasnik : official publication of the Medical Association of Zenica-Doboj Canton, Bosnia and Herzegovina 2020 aug
In vitro regulation of IL-6 and TGF-\ss by mesenchymal stem cells in systemic lupus erythematosus patients.
D. Masyithah Darlan et al.
Abstract
Aim To analyse the ability of mesenchymal stem cells (MSCs) to regulate interleukin 6 (IL-6) and transforming growth factor (TGF-$\beta$) expression in vitro under co-culture conditions in human systemic lupus erythematosus (SLE). Method This study used a post-test group design that used peripheral blood mononuclear cells (PBMCs) from SLE patients at Kariadi Hospital, Semarang, Indonesia, and MSCs from a human umbilical cord. The cells were divided into two groups. The control group of PBMCs was treated with a standard medium, and the treatment group was co-cultured with the MSCs at a 1:40 ratio. Following 24 h incubation, the levels of IL-6 and TGF-$\beta$ released in the culture medium were measured using a specific ELISA assay. Results This study showed a significant decrease in IL-6 level (p{\textless}0.05) and a significant increase in TGF-$\beta$ level (p{\textless}0.001) following 24 h of co-culture incubation of human SLE PBMCs cells and MSCs. Conclusion The PBMCs-to-MSCs ratio of 1:40 can regulate the IL-6 and TGF-$\beta$ levels in human SLE PBMCs.
Stem cells international 2020
Human Supernumerary Teeth-Derived Apical Papillary Stem Cells Possess Preferable Characteristics and Efficacy on Hepatic Fibrosis in Mice.
J. Yao et al.
Abstract
Dental tissue has been acknowledged as an advantaged source for high-quality dental pulp stem cell (DPSC) preparation. However, despite the accomplishment of the separation of DPSCs from permanent teeth and supernumerary teeth, the deficiency of rigorous and systematic clarification on the signatures and efficacy will hinder their prospects in regenerative medicine. In this study, we primitively isolated permanent teeth-derived DPSCs and supernumerary teeth-derived apical papillary stem cells (SCAP-Ss) with parental consent. Immunophenotype of DPSCs and SCAP-Ss was determined by a flow cytometry assay, and the cell viability was verified by multidimensional detections including cell proliferation, cell cycle, apoptosis, and senescence. The migration and clonogenic capacity were examined by a wound healing test and crystal violet staining, respectively. The multilineage differentiation potential was quantitated by utilizing Oil Red O staining and Alizarin Red staining, together with real-time PCR analysis. The efficacy on a mouse hepatic fibrosis model was evaluated by using histologic sections and liver function tests. Herein, we showed that SCAP-Ss exhibited comparable immunophenotype and adipogenic differentiation capacity as DPSCs. However, different from DPSCs, SCAP-Ss exhibited superiority in cell viability and osteogenic differentiation. Simultaneously, injection of DPSCs and SCAP-Ss significantly reduced inflammatory infiltration, enhanced liver-associated gene expression, and finally relieved symptoms of hepatic fibrosis. In conclusion, SCAP-Ss possess preferable characteristics and efficacy on hepatic fibrosis in mice. Our findings suggest that SCAP-Ss are an easily accessible postnatal stem cell source with multifaceted characteristics for regenerative medicine.
View All Publications