MesenCult™ Adipogenic Differentiation Kit (Human)
Medium for the differentiation of human MSCs into adipocytes
概要
MesenCult™ Adipogenic Differentiation Medium (Human) is specifically formulated for the in vitro differentiation of human mesenchymal stromal cells (also known as mesenchymal stem cells or MSCs) into adipogenic lineage cells. This kit is suitable for the differentiation of MSCs derived from human bone marrow (BM), adipose tissue, umbilical cord, or pluripotent stem cells (PSCs) that have been previously culture-expanded in serum- and animal component-free medium (e.g. MesenCult™ ACF Plus Medium [Catalog #05445]), serum-containing medium (e.g. MesenCult™ Proliferation Kit [Catalog #05411]), or platelet lysate medium (e.g. MesenCult™-hPL Medium [Catalog #05439]).
Advantages
• Robust and versatile human MSC differentiation to adipocytes
• Optimized for differentiation of bone marrow- and adipose tissue-derived human MSCs previously cultured in serum-containing or serum-free media, such as MesenCult™-ACF Plus Medium
• Compatible with human MSCs previously cultured in platelet lysate media
• Optimized for differentiation of bone marrow- and adipose tissue-derived human MSCs previously cultured in serum-containing or serum-free media, such as MesenCult™-ACF Plus Medium
• Compatible with human MSCs previously cultured in platelet lysate media
Components
- MesenCult™ MSC Basal Medium (Human), 225 mL
- MesenCult™ 10X Adipogenic Differentiation Supplement (Human), 25 mL
- MesenCult™ 500X Adipogenic Differentiation Supplement (Human), 0.5 mL
Subtype
Specialized Media
Cell Type
Adipocytes, Mesenchymal Stem and Progenitor Cells
Species
Human
Application
Cell Culture, Differentiation
Brand
MesenCult
Area of Interest
Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MesenCult™ Adipogenic Differentiation Kit (Human) | 05412 | All | English |
| Safety Data Sheet 1 | MesenCult™ Adipogenic Differentiation Kit (Human) | 05412 | All | English |
| Safety Data Sheet 2 | MesenCult™ Adipogenic Differentiation Kit (Human) | 05412 | All | English |
| Safety Data Sheet 3 | MesenCult™ Adipogenic Differentiation Kit (Human) | 05412 | All | English |
数据及文献
Data
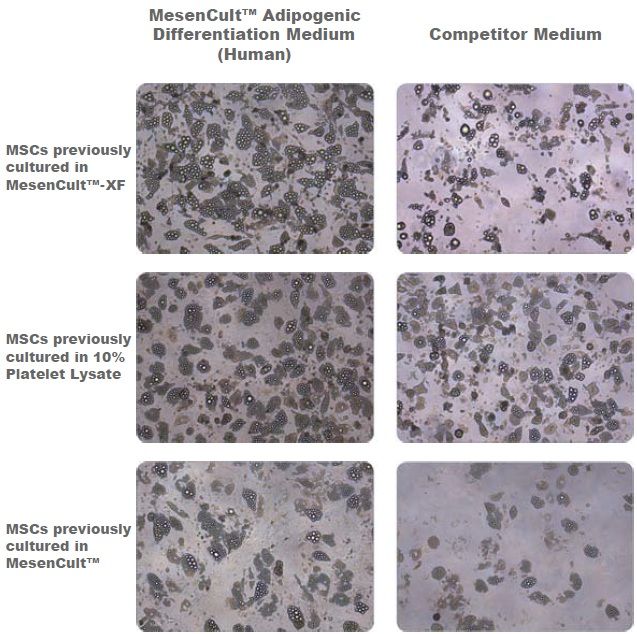
Figure 1. Adipogenic Differentiation of Human Bone Marrow-Derived MSCs
Adipogenic differentiation of human bone marrow-derived MSCs using MesenCult™ Adipogenic Differentiation Medium (Human) or a competitor medium. Prior to differentiation, MSCs were cultured for 2 passages in either serum- and xeno-free media (MesenCult™-XF or a 10% platelet lysate-based formulation) or serum-containing medium (MesenCult™) before undergoing differentiation. Even though differentation results are donor dependent, MesenCult™ Adipogenic Differentiation Medium (Human) consistently performed as well as, or better than the competitor medium. This trend was consistent for MSCs previously cultured in MesenCult™-XF, 10% Platelet Lysate or MesenCult™ medium.
Publications (13)
Clinical and experimental dental research 2020 may
Differential expression of drug resistance genes in CD146 positive dental pulp derived stem cells and CD146 negative fibroblasts.
Abstract
Abstract
INTRODUCTION The stem cell portion of the dental pulp derived cultures (DPSCs) showed a higher resistance to cytotoxic effect of restorative dental materials compared to pulpal fibroblasts (DPFs). Here, we aimed to compare the expression of some drug resistant genes between these cells. METHODS AND MATERIALS To separate DPSCs from DPFs, we used magnetic cell sorting technique based on CD146 expression. To assess the stem cell properties, the positive and negative portions underwent colony forming assays and were induced to be differentiated into the adipocytes, osteoblasts, hepatocytes, and neural cells. Cell surface antigen panels were checked using immune fluorescence and flow-cytometry techniques. The mRNA expression of 14 ABC transporters including ABCA2, ABCB1, ABCB11, ABCC1, ABCC2, ABCC3, ABCC4, ABCC5-2, ABCC5-4,ABCC5-13, ABCC6, ABCC10, ABCC11, and ABCG2 genes was assessed, using quantitative RT-PCR technique. RESULTS Only the CD146 positive portion could be differentiated into the desired fates, and they formed higher colonies (16.7 ± 3.32 vs. 1.7 ± 1.67, p {\textless} .001). The cell surface antigen panels were the same, except for CD146 and STRO-1 markers which were expressed only in the positive portion. Among the ABC transporter genes studied, the positive portion showed a higher expression (approximately two-fold) of ABCA2, ABCC5-13, and ABCC5-2 genes. CONCLUSION Dental pulp stem cells which can be separated from dental pulp fibroblasts based on CD146 expression, express higher levels of some drug resistance genes which probably accounts for their features of more resistance to cytotoxic effects of some dental materials. This needs to be more validated in future.
Science advances 2020 may
In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion.
Abstract
Abstract
Increasing occurrence of moderate to severe intrauterine adhesion (IUA) is seriously affecting the quality of human life. The aim of the study was to establish IUA models in nonhuman primates and to explore the dual repair effects of human umbilical cord-derived mesenchymal stem cells (huMSCs) loaded on autocrosslinked hyaluronic acid gel (HA-GEL) on endometrial damage and adhesion. Here, we recorded the menstrual cycle data in detail with uterine cavities observed and endometrial tissues detected after intervention, and the thicker endometria, decreased amount of fibrotic formation, increased number of endometrium glands, etc., suggested that both HA-GEL and huMSC/HA-GEL complexes could partially repair IUA caused by mechanical injury, but huMSC/HA-GEL complex transplantation had notable dual repair effects: a reliable antiadhesion property and the promotion of endometrial regeneration.
Journal of cellular and molecular medicine 2020 mar
Intravenous administration of iPS-MSCSPIONs mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat.
Abstract
Abstract
This study traced intravenously administered induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (MSC) and assessed the impact of iPSC-MSC on preserving renal function in SD rat after 5/6 nephrectomy. The results of in vitro study showed that FeraTrack™Direct contrast particles (ie intracellular magnetic labelling) in the iPSC-MSC (ie iPS-MSCSPIONs ) were clearly identified by Prussian blue stain. Adult-male SD rats (n = 40) were categorized into group 1 (SC), group 2 [SC + iPS-MSCSPIONs (1.0 × 106 cells)/intravenous administration post-day-14 CKD procedure], group 3 (CKD), group 4 [CKD + iPS-MSCSPIONs (0.5 × 106 cells)] and group 5 [CKD + iPS-MSCSPIONs (1.0 × 106 cells)]. By day-15 after CKD induction, abdominal MRI demonstrated that iPS-MSCSPIONs were only in the CKD parenchyma of groups 4 and 5. By day 60, the creatinine level/ratio of urine protein to urine creatinine/kidney injury score (by haematoxylin and eosin stain)/fibrotic area (Masson's trichrome stain)/IF microscopic finding of kidney injury molecule-1 expression was lowest in groups 1 and 2, highest in group 3, and significantly higher in group 4 than in group 5, whereas IF microscopic findings of podocyte components (ZO-1/synaptopodin) and protein levels of anti-apoptosis ((Bad/Bcl-xL/Bcl-2) exhibited an opposite pattern to creatinine level among the five groups (all P {\textless} .0001). The protein expressions of cell-proliferation signals (PI3K/p-Akt/m-TOR, p-ERK1/2, FOXO1/GSK3$\beta$/p90RSK), apoptotic/DNA-damage (Bax/caspases8-10/cytosolic-mitochondria) and inflammatory (TNF-$\alpha$/TNFR1/TRAF2/NF-$\kappa$B) biomarkers displayed an identical pattern to creatinine level among the five groups (all P {\textless} .0001). The iPS-MSCSPIONs that were identified only in CKD parenchyma effectively protected the kidney against CKD injury.
Stem cells and development 2020 jul
Human Umbilical Cord Mesenchymal Stem Cells Attenuate Abdominal Aortic Aneurysm Progression in Sprague-Dawley Rats: Implication of Vascular Smooth Muscle Cell Phenotypic Modulation.
Abstract
Abstract
Abdominal aortic aneurysm (AAA) is life-threatening, for which efficient nonsurgical treatment strategy has not been available so far. Several previous studies investigating the therapeutic effect of mesenchymal stem cells (MSCs) in AAA indicated that MSCs could inhibit aneurysmal inflammatory responses and extracellular matrix destruction, and suppress aneurysm occurrence and expansion. Vascular smooth muscle cell (VSMC) phenotypic plasticity is reported to be predisposed in AAA initiation and progression. However, little is known about the effect of MSCs on VSMC phenotypic modulation in AAA. In this study, we investigate the therapeutic efficacy of umbilical cord mesenchymal stem cells (UC-MSCs) in elastase-induced AAA model and evaluate the effect of UC-MSC on VSMC phenotypic regulation. We demonstrate that the intravenous injection of UC-MSC attenuates elastase-induced aneurysmal expansion, reduces elastin degradation and fragmentation, inhibits MMPs and TNF-$\alpha$ expression, and preserves and/or restores VSMC contractile phenotype in AAA. Taken together, these results highlight the therapeutic and VSMC phenotypic modulation effects of UC-MSC in AAA progression, which further indicates the potential of applying UC-MSC as an alternative treatment candidate for AAA.
Bone research 2020
Comparison of skeletal and soft tissue pericytes identifies CXCR4+ bone forming mural cells in human tissues.
Abstract
Abstract
Human osteogenic progenitors are not precisely defined, being primarily studied as heterogeneous multipotent cell populations and termed mesenchymal stem cells (MSCs). Notably, select human pericytes can develop into bone-forming osteoblasts. Here, we sought to define the differentiation potential of CD146+ human pericytes from skeletal and soft tissue sources, with the underlying goal of defining cell surface markers that typify an osteoblastogenic pericyte. CD146+CD31-CD45- pericytes were derived by fluorescence-activated cell sorting from human periosteum, adipose, or dermal tissue. Periosteal CD146+CD31-CD45- cells retained canonical features of pericytes/MSC. Periosteal pericytes demonstrated a striking tendency to undergo osteoblastogenesis in vitro and skeletogenesis in vivo, while soft tissue pericytes did not readily. Transcriptome analysis revealed higher CXCR4 signaling among periosteal pericytes in comparison to their soft tissue counterparts, and CXCR4 chemical inhibition abrogated ectopic ossification by periosteal pericytes. Conversely, enrichment of CXCR4+ pericytes or stromal cells identified an osteoblastic/non-adipocytic precursor cell. In sum, human skeletal and soft tissue pericytes differ in their basal abilities to form bone. Diversity exists in soft tissue pericytes, however, and CXCR4+ pericytes represent an osteoblastogenic, non-adipocytic cell precursor. Indeed, enrichment for CXCR4-expressing stromal cells is a potential new tactic for skeletal tissue engineering.
Stem cells international 2020
Human Supernumerary Teeth-Derived Apical Papillary Stem Cells Possess Preferable Characteristics and Efficacy on Hepatic Fibrosis in Mice.
Abstract
Abstract
Dental tissue has been acknowledged as an advantaged source for high-quality dental pulp stem cell (DPSC) preparation. However, despite the accomplishment of the separation of DPSCs from permanent teeth and supernumerary teeth, the deficiency of rigorous and systematic clarification on the signatures and efficacy will hinder their prospects in regenerative medicine. In this study, we primitively isolated permanent teeth-derived DPSCs and supernumerary teeth-derived apical papillary stem cells (SCAP-Ss) with parental consent. Immunophenotype of DPSCs and SCAP-Ss was determined by a flow cytometry assay, and the cell viability was verified by multidimensional detections including cell proliferation, cell cycle, apoptosis, and senescence. The migration and clonogenic capacity were examined by a wound healing test and crystal violet staining, respectively. The multilineage differentiation potential was quantitated by utilizing Oil Red O staining and Alizarin Red staining, together with real-time PCR analysis. The efficacy on a mouse hepatic fibrosis model was evaluated by using histologic sections and liver function tests. Herein, we showed that SCAP-Ss exhibited comparable immunophenotype and adipogenic differentiation capacity as DPSCs. However, different from DPSCs, SCAP-Ss exhibited superiority in cell viability and osteogenic differentiation. Simultaneously, injection of DPSCs and SCAP-Ss significantly reduced inflammatory infiltration, enhanced liver-associated gene expression, and finally relieved symptoms of hepatic fibrosis. In conclusion, SCAP-Ss possess preferable characteristics and efficacy on hepatic fibrosis in mice. Our findings suggest that SCAP-Ss are an easily accessible postnatal stem cell source with multifaceted characteristics for regenerative medicine.