MesenCult™-ACF Plus Medium
Animal component-free medium for human mesenchymal stem cells
概要
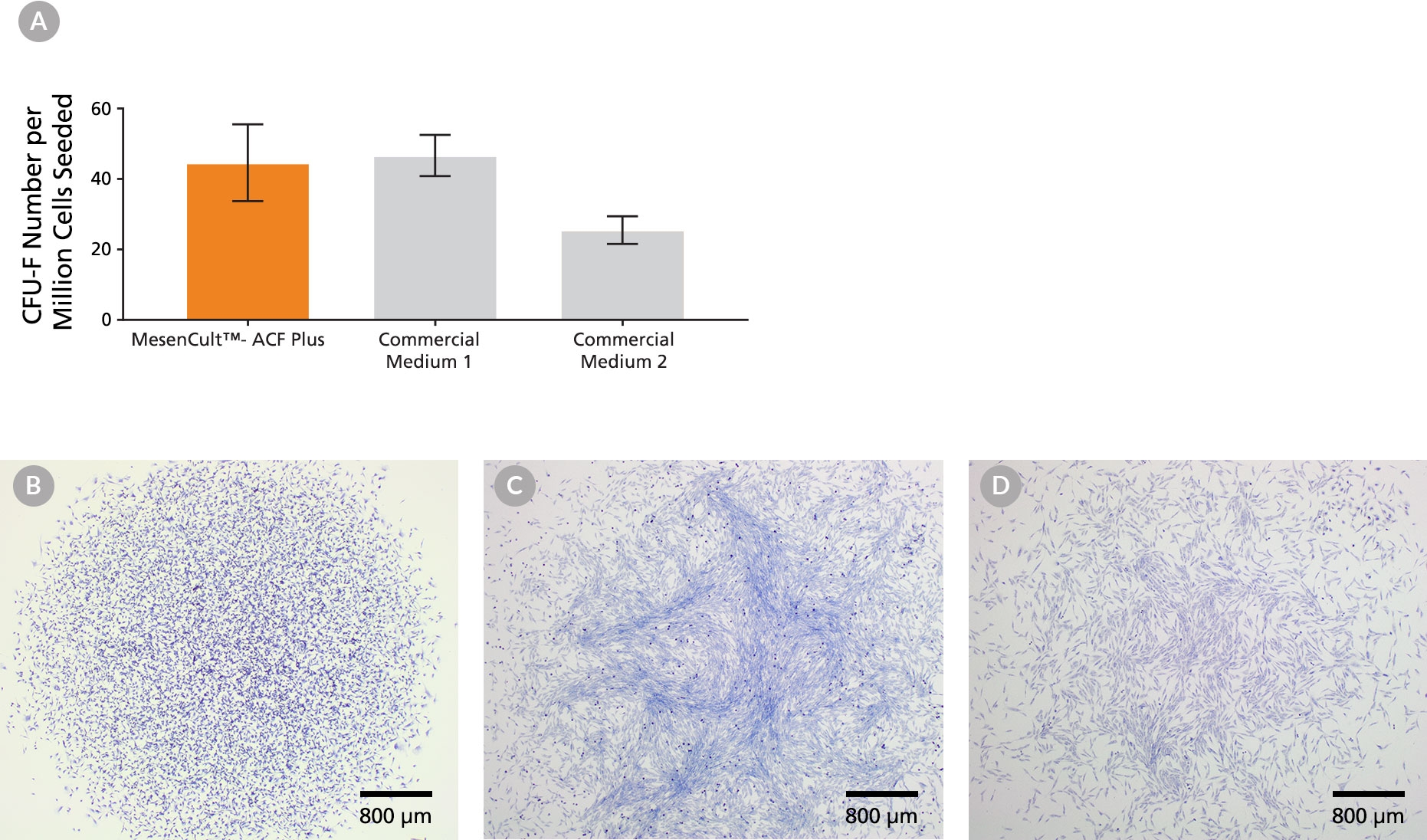
MesenCult™-ACF Plus Medium is a standardized, animal component-free (ACF) and serum-free medium for the isolation and culture of human mesenchymal stromal cells, also known as mesenchymal stem cells (MSCs). MesenCult™-ACF Plus Medium is optimized for the expansion of MSCs in vitro as well as their enumeration using the colony-forming unit-fibroblast (CFU-F) assay. MesenCult™-ACF Plus Medium supports the isolation and long-term growth of human bone marrow-derived MSCs, and cells maintain robust multi-lineage differentiation potential in vitro.
MesenCult™-ACF Plus Medium must be used in conjunction with Animal Component-Free Cell Attachment Substrate (Component #07130) and Animal Component-Free Cell Dissociation Kit (Catalog #05426), providing a complete, defined ACF culture system. Components of Animal Component-Free Cell Attachment Substrate and Animal Component-Free Cell Dissociation Kit are pre-screened and tested for optimal cell adherence when cells are cultured with MesenCult™-ACF Plus Medium.
For animal component-free and optimized cryopreservation, MesenCult™-ACF Freezing Medium (Catalog #05490) is recommended for human MSCs previously cultured in MesenCult™ media, including MesenCult™-ACF Plus. For a complete list of related products, including differentiation media available, visit www.stemcell.com or contact us at techsupport@stemcell.com.
NOTE: Complete MesenCult™-ACF Plus Medium must be supplemented with L-Glutamine (Catalog #07100).
• Animal component-free formulation improves experimental reproducibility.
• Superior cell expansion compared to serum-containing media.
• Cultured MSCs retain robust expansion and tri-lineage differentiation capacities at early and late passages.
• Supports MSC derivation directly from primary human tissue.
MesenCult™-ACF Plus Medium Kit (Catalog #05445)
• MesenCult™-ACF Plus Medium, 500 mL
• MesenCult™-ACF Plus 500X Supplement, 1 mL
MesenCult™-ACF Plus Culture Kit (Catalog #05448)
• MesenCult™-ACF Plus Medium, 500 mL
• MesenCult™-ACF Plus 500X Supplement, 1 mL
• Animal Component-Free Cell Attachment Substrate, 1 mL
Mesenchymal Cells, PSC-Derived, Mesenchymal Stem and Progenitor Cells
Cell Culture, Expansion, Maintenance
Extracellular Vesicle Research, Stem Cell Biology
数据及文献
Publications (8)
Leukemia 2020 jun
Despite mutation acquisition in hematopoietic stem cells, JMML-propagating cells are not always restricted to this compartment.
A. Caye et al.
Abstract
Juvenile myelomonocytic leukemia (JMML) is a rare aggressive myelodysplastic/myeloproliferative neoplasm of early childhood, initiated by RAS-activating mutations. Genomic analyses have recently described JMML mutational landscape; however, the nature of JMML-propagating cells (JMML-PCs) and the clonal architecture of the disease remained until now elusive. Combining genomic (exome, RNA-seq), Colony forming assay and xenograft studies, we detect the presence of JMML-PCs that faithfully reproduce JMML features including the complex/nonlinear organization of dominant/minor clones, both at diagnosis and relapse. Further integrated analysis also reveals that although the mutations are acquired in hematopoietic stem cells, JMML-PCs are not always restricted to this compartment, highlighting the heterogeneity of the disease during the initiation steps. We show that the hematopoietic stem/progenitor cell phenotype is globally maintained in JMML despite overexpression of CD90/THY-1 in a subset of patients. This study shed new lights into the ontogeny of JMML, and the identity of JMML-PCs, and provides robust models to monitor the disease and test novel therapeutic approaches.
Cell 2020 jun
CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling.
Y. Oguri et al.
Abstract
Adipose tissues dynamically remodel their cellular composition in response to external cues by stimulating beige adipocyte biogenesis; however, the developmental origin and pathways regulating this process remain insufficiently understood owing to adipose tissue heterogeneity. Here, we employed single-cell RNA-seq and identified a unique subset of adipocyte progenitor cells (APCs) that possessed the cell-intrinsic plasticity to give rise to beige fat. This beige APC population is proliferative and marked by cell-surface proteins, including PDGFR$\alpha$, Sca1, and CD81. Notably, CD81 is not only a beige APC marker but also required for de novo beige fat biogenesis following cold exposure. CD81 forms a complex with $\alpha$V/$\beta$1 and $\alpha$V/$\beta$5 integrins and mediates the activation of integrin-FAK signaling in response to irisin. Importantly, CD81 loss causes diet-induced obesity, insulin resistance, and adipose tissue inflammation. These results suggest that CD81 functions as a key sensor of external inputs and controls beige APC proliferation and whole-body energy homeostasis.
Stem cells translational medicine 2020 jul
Precision installation of a highly efficient suicide gene safety switch in human induced pluripotent stem cells.
Z.-D. Shi et al.
Abstract
Human pluripotent stem cells including induced pluripotent stem cells (iPSCs) and embryonic stem cells hold great promise for cell-based therapies, but safety concerns that complicate consideration for routine clinical use remain. Installing a safety switch" based on the inducible caspase-9 (iCASP9) suicide gene system should offer added control over undesirable cell replication or activity. Previous studies utilized lentiviral vectors to integrate the iCASP9 system into T cells and iPSCs. This method results in random genomic insertion of the suicide switch and inefficient killing of the cells after the switch is "turned on" with a small molecule (eg AP1903). To improve the safety and efficiency of the iCASP9 system for use in iPSC-based therapy we precisely installed the system into a genomic safe harbor the AAVS1 locus in the PPP1R12C gene. We then evaluated the efficiencies of different promoters to drive iCASP9 expression in human iPSCs. We report that the commonly used EF1$\alpha$ promoter is silenced in iPSCs and that the endogenous promoter of the PPP1R12C gene is not strong enough to drive high levels of iCASP9 expression. However the CAG promoter induces strong and stable iCASP9 expression in iPSCs and activation of this system with AP1903 leads to rapid killing and complete elimination of iPSCs and their derivatives including MSCs and chondrocytes in vitro. Furthermore iPSC-derived teratomas shrank dramatically or were completely eliminated after administration of AP1903 in mice. Our data suggest significant improvements on existing iCASP9 suicide switch technologies and may serve as a guide to other groups seeking to improve the safety of stem cell-based therapies."
International journal of nanomedicine 2020
Topical Application of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells in Combination with Sponge Spicules for Treatment of Photoaging.
K. Zhang et al.
Abstract
Purpose The topical application of exosomes secreted by mesenchymal stem cells (MSC-Exos) on the skin is a very new and interesting topic in the medical field. In this study, we aimed to investigate whether marine sponge Haliclona sp. spicules (SHSs) could effectively enhance the skin delivery of human umbilical cord-derived MSC-Exos (hucMSC-Exos), and further evaluate the topical application of hucMSC-Exos combined with SHSs in rejuvenating photoaged mouse skin. Materials and Methods SHSs were isolated from the explants of sponge Haliclona sp. with our proprietary method, and hucMSC-Exos were prepared from the conditioned medium of hucMSCs using ultracentrifugation. The effects of SHSs on the skin penetration of fluorescently labeled hucMSC-Exos were determined using confocal microscopy in vitro (porcine skin) and in vivo (mouse skin). The therapeutic effects of hucMSC-Exos coupled with SHSs against UV-induced photoaging in mice were assessed by using microwrinkles analysis, pathohistological examination and real-time RT-PCR. We also tested the skin irritation caused by the combination of hucMSC-Exos and SHSs in guinea pigs. Results In vitro results showed that hucMSC-Exos could not readily penetrate through porcine skin by themselves. However, SHSs increased the skin absorption of exosomes by a factor of 5.87 through creating microchannels. Similar penetration enhancement of hucMSC-Exos was observed after SHSs treatment in mice. The combined use of hucMSC-Exos and SHSs showed significant anti-photoaging effects in mice, including reducing microwrinkles, alleviating histopathological changes, and promoting the expression of extracellular matrix constituents, whereas hucMSC-Exos alone produced considerably weaker effects. Skin irritation test showed that the combination of hucMSC-Exos and SHSs caused slight irritation, and the skin recovered shortly. Conclusion SHSs provide a safe and effective way to enhance the skin delivery of MSC-Exos. Moreover, the combination of MSC-Exos and SHSs may be of much use in the treatment of photoaging.
Blood advances 2019 nov
Human models of NUP98-KDM5A megakaryocytic leukemia in mice contribute to uncovering new biomarkers and therapeutic vulnerabilities.
S. Cardin et al.
Abstract
Acute megakaryoblastic leukemia (AMKL) represents ∼10{\%} of pediatric acute myeloid leukemia cases and typically affects young children ({\textless}3 years of age). It remains plagued with extremely poor treatment outcomes ({\textless}40{\%} cure rates), mostly due to primary chemotherapy refractory disease and/or early relapse. Recurrent and mutually exclusive chimeric fusion oncogenes have been detected in 60{\%} to 70{\%} of cases and include nucleoporin 98 (NUP98) gene rearrangements, most commonly NUP98-KDM5A. Human models of NUP98-KDM5A-driven AMKL capable of faithfully recapitulating the disease have been lacking, and patient samples are rare, further limiting biomarkers and drug discovery. To overcome these impediments, we overexpressed NUP98-KDM5A in human cord blood hematopoietic stem and progenitor cells using a lentiviral-based approach to create physiopathologically relevant disease models. The NUP98-KDM5A fusion oncogene was a potent inducer of maturation arrest, sustaining long-term proliferative and progenitor capacities of engineered cells in optimized culture conditions. Adoptive transfer of NUP98-KDM5A-transformed cells into immunodeficient mice led to multiple subtypes of leukemia, including AMKL, that phenocopy human disease phenotypically and molecularly. The integrative molecular characterization of synthetic and patient NUP98-KDM5A AMKL samples revealed SELP, MPIG6B, and NEO1 as distinctive and novel disease biomarkers. Transcriptomic and proteomic analyses pointed to upregulation of the JAK-STAT signaling pathway in the model AMKL. Both synthetic models and patient-derived xenografts of NUP98-rearranged AMKL showed in vitro therapeutic vulnerability to ruxolitinib, a clinically approved JAK2 inhibitor. Overall, synthetic human AMKL models contribute to defining functional dependencies of rare genotypes of high-fatality pediatric leukemia, which lack effective and rationally designed treatments.
Stem cells international 2019
Gestational Tissue-Derived Human Mesenchymal Stem Cells Use Distinct Combinations of Bioactive Molecules to Suppress the Proliferation of Human Hepatoblastoma and Colorectal Cancer Cells.
N. Paiboon et al.
Abstract
Background Cancer has been considered a serious global health problem and a leading cause of morbidity and mortality worldwide. Despite recent advances in cancer therapy, treatments of advance stage cancers are mostly ineffective resulting in poor survival of patients. Recent evidences suggest that multipotent human mesenchymal stem cells (hMSCs) play important roles in growth and metastasis of several cancers by enhancing their engraftment and inducing tumor neovascularization. However, the effect of hMSCs on cancer cells is still controversial because there are also evidences demonstrating that hMSCs inhibited growth and metastasis of some cancers. Methods In this study, we investigated the effects of bioactive molecules released from bone marrow and gestational tissue-derived hMSCs on the proliferation of various human cancer cells, including C3A, HT29, A549, Saos-2, and U251. We also characterized the hMSC-derived factors that inhibit cancer cell proliferation by protein fractionation and mass spectrometry analysis. Results We herein make a direct comparison and show that the effects of hMSCs on cancer cell proliferation and migration depend on both hMSC sources and cancer cell types and cancer-derived bioactive molecules did not affect the cancer suppressive capacity of hMSCs. Moreover, hMSCs use distinct combination of bioactive molecules to suppress the proliferation of human hepatoblastoma and colorectal cancer cells. Using protein fractionation and mass spectrometry analysis, we have identified several novel hMSC-derived factors that might be able to suppress cancer cell proliferation. Conclusion We believe that the procedure developed in this study could be used to discover other therapeutically useful molecules released by various hMSC sources for a future in vivo study.
View All Publications