MesenCult™-ACF Chondrogenic Differentiation Kit
Animal component-free medium for the differentiation of MSCs into chondrocytes
概要
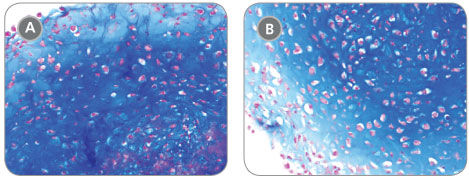
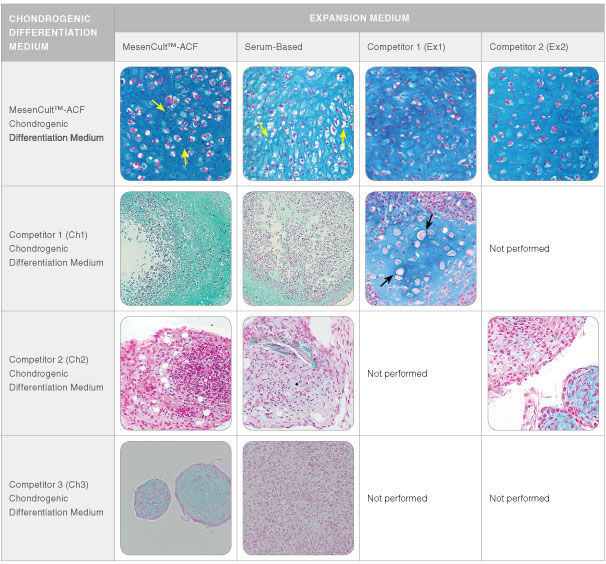
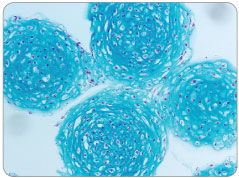
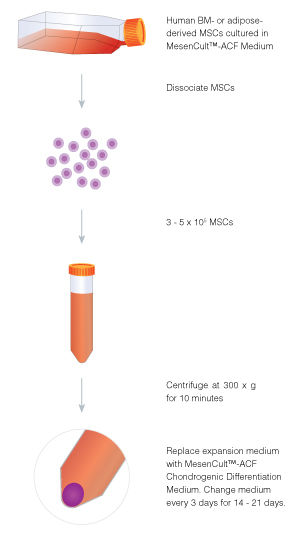
MesenCult™-ACF Chondrogenic Differentiation Medium is animal component-free (ACF) and specifically formulated for the in vitro differentiation of human mesenchymal stromal cells (MSCs; also known as mesencyhymal stem cells) into chondrogenic lineage cells, including chondrocytes. This medium is suitable for the differentiation of human bone marrow (BM)-, adipose tissue (AT)- and synovium (S)-derived MSCs previously culture-expanded in serum-containing medium (e.g. MesenCult™ Proliferation Kit [Catalog #05411]) or animal component-free MesenCult™-ACF Plus Medium [Catalog #05445]). MesenCult™-ACF Chondrogenic Differentiation Medium induces robust chondrogenic differentiation of human MSCs with as few as 3 x 10^5 cells and as early as day 14.
• Animal component-free (ACF) formulation
• Robust chondrogenic differentiation with as few as 3 x 10^5 MSCs and as early as day 14
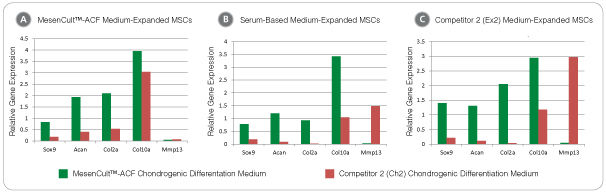
• Strong expression of chondrogenic transcripts - Acan, Col2a, Sox9 and Col10a; low expression of hypertrophic transcript Mmp13
• Completes optimized ACF workflow for MSC isolation, expansion, cryopreservation and chondrogenic differentiation
- MesenCult™-ACF Chondrogenic Differentiation Basal Medium, 95 mL
- MesenCult™-ACF 20X Chondrogenic Differentiation Supplement, 5 mL
Chondrocytes, Mesenchymal Stem and Progenitor Cells
Cell Culture, Differentiation
数据及文献
Publications (11)
Stem cells and development 2020 jul
Human Umbilical Cord Mesenchymal Stem Cells Attenuate Abdominal Aortic Aneurysm Progression in Sprague-Dawley Rats: Implication of Vascular Smooth Muscle Cell Phenotypic Modulation.
H. Wen et al.
Abstract
Abdominal aortic aneurysm (AAA) is life-threatening, for which efficient nonsurgical treatment strategy has not been available so far. Several previous studies investigating the therapeutic effect of mesenchymal stem cells (MSCs) in AAA indicated that MSCs could inhibit aneurysmal inflammatory responses and extracellular matrix destruction, and suppress aneurysm occurrence and expansion. Vascular smooth muscle cell (VSMC) phenotypic plasticity is reported to be predisposed in AAA initiation and progression. However, little is known about the effect of MSCs on VSMC phenotypic modulation in AAA. In this study, we investigate the therapeutic efficacy of umbilical cord mesenchymal stem cells (UC-MSCs) in elastase-induced AAA model and evaluate the effect of UC-MSC on VSMC phenotypic regulation. We demonstrate that the intravenous injection of UC-MSC attenuates elastase-induced aneurysmal expansion, reduces elastin degradation and fragmentation, inhibits MMPs and TNF-$\alpha$ expression, and preserves and/or restores VSMC contractile phenotype in AAA. Taken together, these results highlight the therapeutic and VSMC phenotypic modulation effects of UC-MSC in AAA progression, which further indicates the potential of applying UC-MSC as an alternative treatment candidate for AAA.
JCI insight 2020 jul
Endogenous CCN family member WISP1 inhibits trauma-induced heterotopic ossification.
G. C.-Y. Hsu et al.
Abstract
Heterotopic ossification (HO) is defined as abnormal differentiation of local stromal cells of mesenchymal origin, resulting in pathologic cartilage and bone matrix deposition. Cyr61, CTGF, Nov (CCN) family members are matricellular proteins that have diverse regulatory functions on cell proliferation and differentiation, including the regulation of chondrogenesis. However, little is known regarding CCN family member expression or function in HO. Here, a combination of bulk and single-cell RNA sequencing defined the dynamic temporospatial pattern of CCN family member induction within a mouse model of trauma-induced HO. Among CCN family proteins, Wisp1 (also known as Ccn4) was most upregulated during the evolution of HO, and Wisp1 expression corresponded with chondrogenic gene profile. Immunohistochemistry confirmed WISP1 expression across traumatic and genetic HO mouse models as well as in human HO samples. Transgenic Wisp1LacZ/LacZ knockin animals showed an increase in endochondral ossification in HO after trauma. Finally, the transcriptome of Wisp1-null tenocytes revealed enrichment in signaling pathways, such as the STAT3 and PCP signaling pathways, that may explain increased HO in the context of Wisp1 deficiency. In sum, CCN family members, and in particular Wisp1, are spatiotemporally associated with and negatively regulate trauma-induced HO formation.
Biomedicine {\&} pharmacotherapy = Biomedecine {\&} pharmacotherapie 2020 jan
Bone marrow mesenchymal stem cells alleviate the daunorubicin-induced subacute myocardial injury in rats through inhibiting infiltration of T lymphocytes and antigen-presenting cells.
Q. Chen et al.
Abstract
INTRODUCTION Bone marrow mesenchymal stem cells (BMSCs) have been extensively investigated from a perspective on cardiac regeneration therapy. The current study aimed to investigate the protective effect conferred by BMSCs in subacute myocardial injury, and to identify an appropriate BMSC reinfusion time. METHODS BMSCs were isolated from human bone marrow blood. Daunorubicin (DNR)-induced subacute myocardial models were subsequently established. The rats with DNR-induced subacute myocardial injury were injected with dexrazoxane (DZR) and/or BMSCs at varying time points, after which cardiac function was evaluated by assessing left ventricular ejection fraction (LVEF) and fraction shortening (FS). The myocardial structural changes were analyzed, after which the levels of CD3 and human leukocyte antigen DR (HLA-DR) were examined to further validate the mechanism by which BMSCs could influence subacute myocardial injury. RESULTS BMSCs combined with DZR treatment enhanced the cardiac function of rats with DNR-induced myocardial injury, as reflected by increased LVEF and FS. DNR-induced myocardial injuries were mitigated via the application of BMSCs combined with treatment of DZR, accompanied by diminished infiltration or vacuolization. Moreover, BMSCs were observed to alleviate infiltration of T lymphocyte and antigen-presenting cells, as evidenced by reduced expression of CD3 and HLA-DR. CONCLUSION Taken together, this study demonstrates that BMSCs could protect against DNR-induced myocardial injury, especially in the first three days of DNR administration. BMSCs combined with DZR exert a better therapeutic effect, but there are individual differences.
Scientific reports 2020 feb
Thalidomide Inhibits Human iPSC Mesendoderm Differentiation by Modulating CRBN-dependent Degradation of SALL4.
D. G. Belair et al.
Abstract
Exposure to thalidomide during a critical window of development results in limb defects in humans and non-human primates while mice and rats are refractory to these effects. Thalidomide-induced teratogenicity is dependent on its binding to cereblon (CRBN), the substrate receptor of the Cul4A-DDB1-CRBN-RBX1 E3 ubiquitin ligase complex. Thalidomide binding to CRBN elicits subsequent ubiquitination and proteasomal degradation of CRBN neosubstrates including SALL4, a transcription factor of which polymorphisms phenocopy thalidomide-induced limb defects in humans. Herein, thalidomide-induced degradation of SALL4 was examined in human induced pluripotent stem cells (hiPSCs) that were differentiated either to lateral plate mesoderm (LPM)-like cells, the developmental ontology of the limb bud, or definitive endoderm. Thalidomide and its immunomodulatory drug (IMiD) analogs, lenalidomide, and pomalidomide, dose-dependently inhibited hiPSC mesendoderm differentiation. Thalidomide- and IMiD-induced SALL4 degradation can be abrogated by CRBN V388I mutation or SALL4 G416A mutation in hiPSCs. Genetically modified hiPSCs expressing CRBN E377V/V388I mutant or SALL4 G416A mutant were insensitive to the inhibitory effects of thalidomide, lenalidomide, and pomalidomide on LPM differentiation while retaining sensitivity to another known limb teratogen, all-trans retinoic acid (atRA). Finally, disruption of LPM differentiation by atRA or thalidomide perturbed subsequent chondrogenic differentiation in vitro. The data here show that thalidomide, lenalidomide, and pomalidomide affect stem cell mesendoderm differentiation through CRBN-mediated degradation of SALL4 and highlight the utility of the LPM differentiation model for studying the teratogenicity of new CRBN modulating agents.
Bioactive materials 2020 dec
An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration.
W. Zhang et al.
Abstract
Endogenous repair of osteochondral defect is usually limited by the insufficient number of cells in the early stage and incomplete cell differentiation in the later stage. The development of drug delivery systems for sequential release of pro-migratory and pro-chondrogenic molecules to induce endogenous bone marrow-derived mesenchymal stem cells (BMSCs) recruitment and chondrogenic differentiation is highly desirable for in situ osteochondral regeneration. In this study, a novel, all-silk-derived sequential delivery system was fabricated by incorporating the tunable drug-loaded silk fibroin (SF) nanospheres into a SF porous matrix. The loading efficiency and release kinetics of biomolecules depended on the initial SF/polyvinyl alcohol (PVA) concentrations (0.2{\%}, 1{\%} and 5{\%}) of the nanospheres, as well as the hydrophobicity of the loaded molecules, resulting in controllable and programmed delivery profiles. Our findings indicated that the 5{\%} nanosphere-incorporated matrix showed a rapid release of E7 peptide during the first 120 h, whereas the 0.2{\%} nanosphere-incorporated matrix provided a slow and sustained release of Kartogenin (KGN) longer than 30 days. During in vitro culture of BMSCs, this functional SF matrix incorporated with E7/KGN nanospheres showed good biocompatibility, as well as enhanced BMSCs migration and chondrogenic differentiation through the release of E7 and KGN. Furthermore, when implanted into rabbit osteochondral defect, the SF nanosphere matrix with sequential E7/KGN release promoted the regeneration of both cartilage and subchondral bone. This work not only provided a novel all-silk-derived drug delivery system for sequential release of molecules, but also a functional tissue-engineered scaffold for osteochondral regeneration.
Bone research 2020
Comparison of skeletal and soft tissue pericytes identifies CXCR4+ bone forming mural cells in human tissues.
J. Xu et al.
Abstract
Human osteogenic progenitors are not precisely defined, being primarily studied as heterogeneous multipotent cell populations and termed mesenchymal stem cells (MSCs). Notably, select human pericytes can develop into bone-forming osteoblasts. Here, we sought to define the differentiation potential of CD146+ human pericytes from skeletal and soft tissue sources, with the underlying goal of defining cell surface markers that typify an osteoblastogenic pericyte. CD146+CD31-CD45- pericytes were derived by fluorescence-activated cell sorting from human periosteum, adipose, or dermal tissue. Periosteal CD146+CD31-CD45- cells retained canonical features of pericytes/MSC. Periosteal pericytes demonstrated a striking tendency to undergo osteoblastogenesis in vitro and skeletogenesis in vivo, while soft tissue pericytes did not readily. Transcriptome analysis revealed higher CXCR4 signaling among periosteal pericytes in comparison to their soft tissue counterparts, and CXCR4 chemical inhibition abrogated ectopic ossification by periosteal pericytes. Conversely, enrichment of CXCR4+ pericytes or stromal cells identified an osteoblastic/non-adipocytic precursor cell. In sum, human skeletal and soft tissue pericytes differ in their basal abilities to form bone. Diversity exists in soft tissue pericytes, however, and CXCR4+ pericytes represent an osteoblastogenic, non-adipocytic cell precursor. Indeed, enrichment for CXCR4-expressing stromal cells is a potential new tactic for skeletal tissue engineering.
View All Publications