ImmunoCult™ Dendritic Cell Culture Kit
ImmunoCult™-ACF Dendritic Cell Medium is a serum-free and animal component-free medium optimized for the in vitro culture and differentiation of human monocytes into DCs.
ImmunoCult™-ACF Dendritic Cell Differentiation Supplement contains a combination of animal component-free recombinant human cytokines formulated to support the differentiation of immature DCs from human monocytes. The ImmunoCult™ Dendritic Cell Maturation Supplement has been formulated to support maturation of immature DCs to mature DCs.
• No need to supplement the medium with serum
• Optimized for differentiation of CD14+ monocytes into immature and subsequently mature DCs
• High yields of mature DCs with the desired phenotype
• Mature DCs are functional, produce the pro-inflammatory cytokine IL-12, and induce T cell proliferation
Data
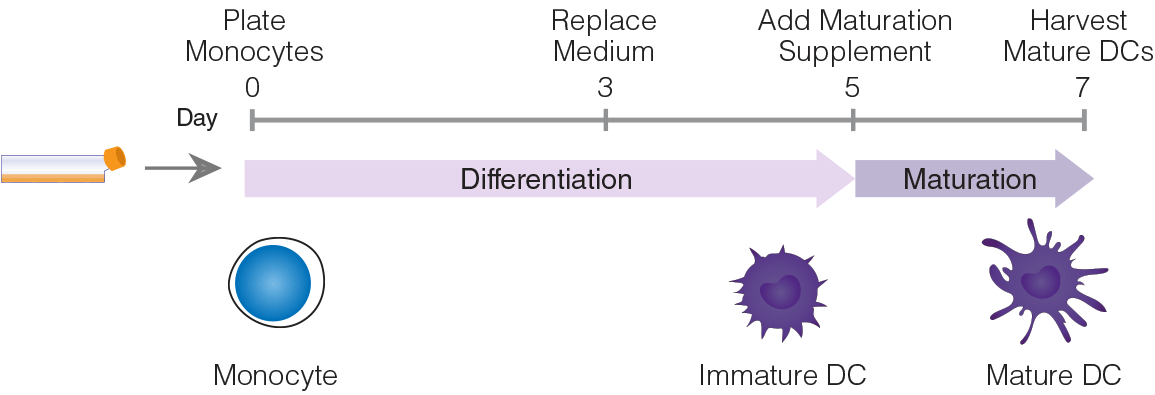
Figure 1. Protocol Diagram.
Mature DCs were generated by culturing EasySep™ isolated monocytes at 1 x 106 cells/mL in ImmunoCult™-ACF Dendritic Cell Medium (Catalog #10987) with added ImmunoCult™-ACF Dendritic Cell Differentiation Supplement (Catalog #10988). At day 3, the medium with differentiation supplement was replaced and cells were incubated for 2 more days. At day 5, without changing the medium, ImmunoCult™ Dendritic Cell Maturation Supplement (Catalog #10989) was added to the culture. At day 7, fully mature DCs were harvested for downstream applications.
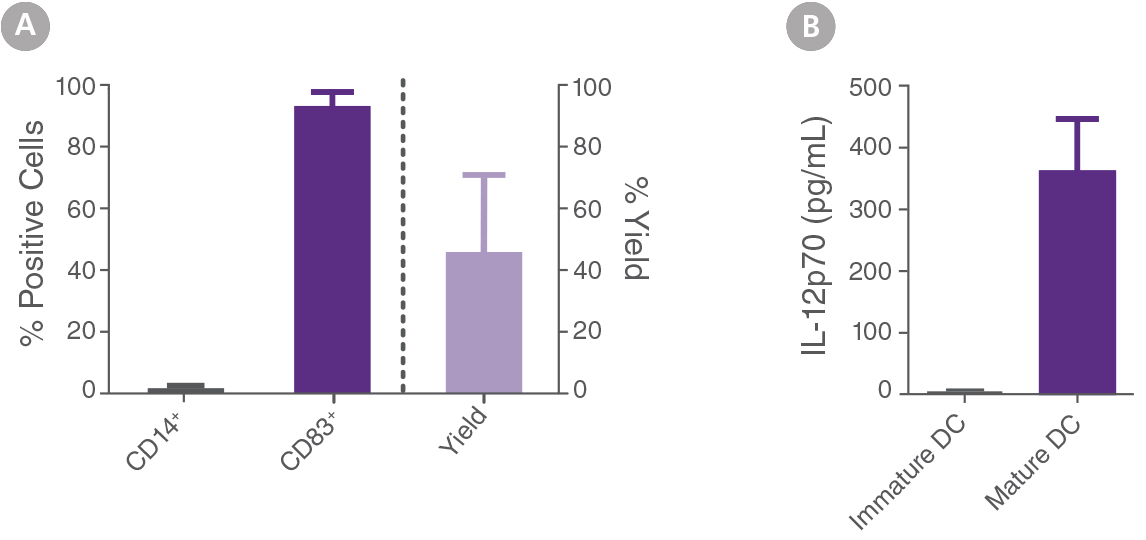
Figure 2. Mature DCs generated with ImmunoCult™-ACF Dendritic Cell Medium with Supplements show desired phenotype.
EasySep™ isolated monocytes were cultured and differentiated into mature DCs as described in Figure 1. (A) The percentage of CD14 and CD83 expression in cells at day 7 (mature DCs) was determined by flow cytometry. At day 7, a total of 93 ± 5% of the cells expressed the mature DC marker CD83 and only 1 ± 1% of cells still expressed the monocyte marker CD14 (mean ± SD, n=39). Yield of mature DCs was determined by count of total viable cells at day 7 relative to the count of viable monocytes used for initial culture at day 0. At day 7, the yield of viable mature DCs corresponded to 45 ± 25% (mean ± SD, n=39). (B) Immature DCs were cultured as described in Figure 1. At day 5, cells were cultured with maturation supplement for 2 days (mature DCs) or without maturation supplement (immature DCs). Supernatant was collected at day 7 and IL-12p70 levels were determined by ELISA. Concentrations of IL-12p70 in supernatant of mature and immature DCs were 361 ± 81 and 5 ± 2 pg/mL, respectively (mean ± SEM, n=27).
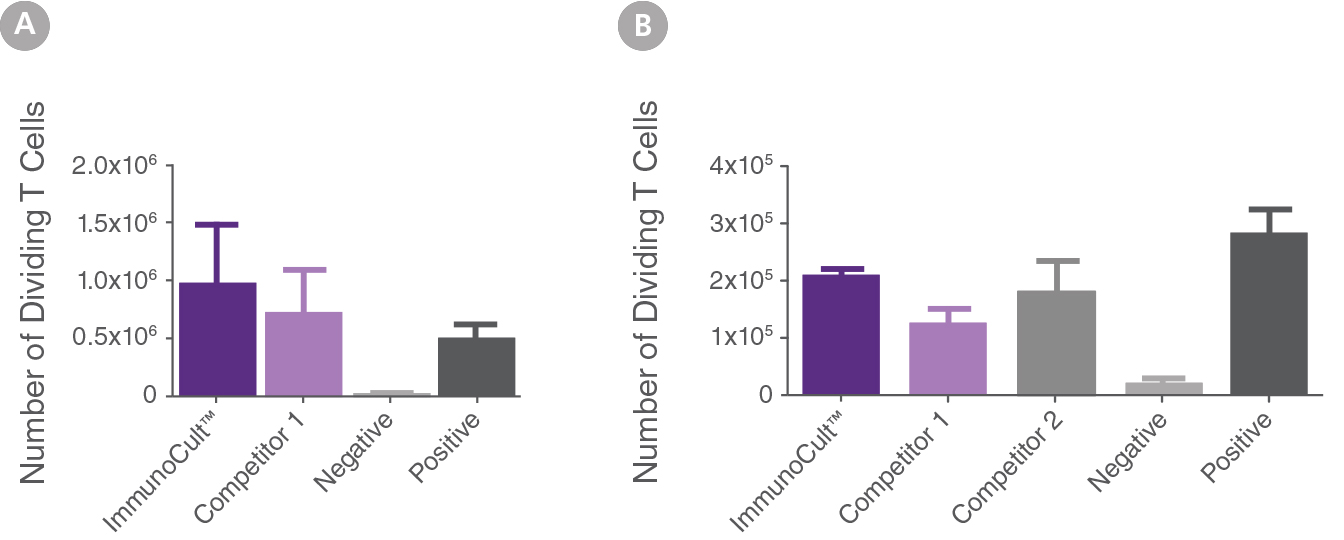
Figure 3. Mature DCs generated with ImmunoCult™-ACF Dendritic Cell Medium and Supplements induce T cell proliferation.
Mature DCs generated with ImmunoCult™-ACF Dendritic Cell Medium and Supplements (ImmunoCult) or other serum-free competitor media (competitor 1 and 2) and corresponding supplements when applicable (competitor 2), were cultured in ImmunoCult™-XF T Cell Expansion Medium with 1 x 105 CFSE labeled (A) allogeneic CD3+ T cells (MLR assay) or (B) autologous CD8+ T cells (antigen-specific T cell response). (A) Cells were cultured at a DC:T cell ratio of 1:25. (B) Prior to culture with T cells, immature DCs were loaded with HLA Class I peptides derived from the human Cytomegalovirus, Epstein-Barr Virus and Influenza Virus (CEF peptide pool) and stimulated with maturation supplement for 2 days. Cells were cultured at a DC:T cell ratio of 1:4 or 1:10. (A,B) CFSE labeled T cells were incubated in media alone (negative control) or with ImmunoCult™ Human CD3/CD28 T Cell Activator (positive control). After 5-7 days in culture the number of dividing T cells ( CD3+CFSElo) was assessed by flow cytometry (mean ± SEM) (A) n=5 (B) n=4 (competitor 1 and 2, n=3). Mature DCs generated in ImmunoCult™-ACF Dendritic Cell Medium induced proliferation of allogeneic and antigen-specific T cells similar to DCs generated in either competitor media. Competitors 1 and 2, include in no particular order, CellGro DC Medium (CellGenix) and PromoCell DC Generation Medium DXF.