IntestiCult™ Organoid Growth Medium (Mouse)
Cell culture medium for establishment and maintenance of mouse intestinal organoids
概要
IntestiCult™ Organoid Growth Medium (Mouse) is a defined, serum-free cell culture medium for efficient establishment and long-term maintenance of mouse intestinal organoids.These organoids, or “mini-guts”, provide a convenient in vitro organotypic culture system for studying both the small and large intestinal epithelium and associated stem cell dynamics. Organoids grown in IntestiCult™ feature a polarized epithelium that contains all of the known cell types of the adult intestinal epithelium. Individual intestinal crypts rapidly form organoids when cultured in IntestiCult™ Organoid Growth Medium (Mouse). Applications of these cultures include studying the development and function of the normal and tumorigenic intestinal epithelium, modeling intestinal disease, and investigating stem cell properties and regenerative therapy approaches. Organoid culture enables convenient in vitro characterization of a system with strong physiological relevance to the adult intestine.
Advantages
• Convenient, in vitro system that recapitulates the identity and organization of the adult intestinal epithelium, including intra- and intercellular signaling, self-propagating stem cell niche and functional transport into and out of the lumen
• Serum-free and defined medium formulation that delivers consistent results
• Enables generation of intestinal organoids in less than one week
• Simple format and easy-to-use protocol
• Serum-free and defined medium formulation that delivers consistent results
• Enables generation of intestinal organoids in less than one week
• Simple format and easy-to-use protocol
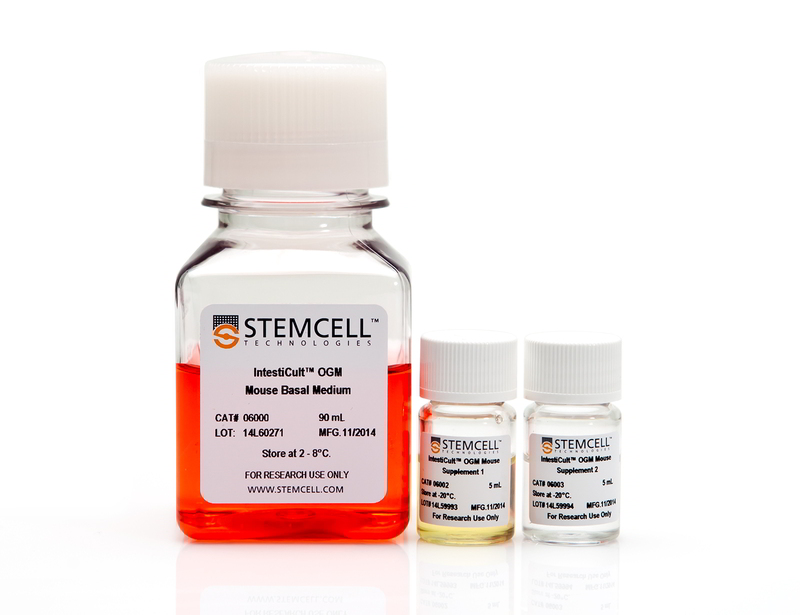
Components
- IntestiCult™ OGM Mouse Basal Medium, 90 mL
- IntestiCult™ OGM Mouse Supplement 1, 5 mL
- IntestiCult™ OGM Mouse Supplement 2, 5 mL
Subtype
Specialized Media
Cell Type
Intestinal Cells
Species
Mouse
Application
Cell Culture, Differentiation, Expansion, Maintenance, Organoid Culture
Brand
IntestiCult
Area of Interest
Cancer Research, Disease Modeling, Drug Discovery and Toxicity Testing, Epithelial Cell Biology, Stem Cell Biology
Formulation
Serum-Free
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | IntestiCult™ Organoid Growth Medium (Mouse) | 06005 | All | English |
| Special Protocol | IntestiCult™ Organoid Growth Medium (Mouse) | 06005 | All | English |
| Safety Data Sheet 1 | IntestiCult™ Organoid Growth Medium (Mouse) | 06005 | All | English |
| Safety Data Sheet 2 | IntestiCult™ Organoid Growth Medium (Mouse) | 06005 | All | English |
| Safety Data Sheet 3 | IntestiCult™ Organoid Growth Medium (Mouse) | 06005 | All | English |
数据及文献
Publications (95)
Cell stem cell 2020 may
Hormonal Suppression of Stem Cells Inhibits Symmetric Cell Division and Gastric Tumorigenesis.
Abstract
Abstract
Cancer is believed to arise from stem cells, but mechanisms that limit the acquisition of mutations and tumor development have not been well defined. We show that a +4 stem cell (SC) in the gastric antrum, marked by expression of Cck2r (a GPCR) and Delta-like ligand 1 (DLL1), is a label-retaining cell that undergoes predominant asymmetric cell division. This +4 antral SC is Notch1low/ Numb+ and repressed by signaling from gastrin-expressing endocrine (G) cells. Chemical carcinogenesis of the stomach is associated with loss of G cells, increased symmetric stem cell division, glandular fission, and more rapid stem cell lineage tracing, a process that can be suppressed by exogenous gastrin treatment. This hormonal suppression is associated with a marked reduction in gastric cancer mutational load, as revealed by exomic sequencing. Taken together, our results show that gastric tumorigenesis is associated with increased symmetric cell division that facilitates mutation and is suppressed by GPCR signaling.
International journal of molecular sciences 2020 may
Iroquois Homeobox Protein 2 Identified as a Potential Biomarker for Parkinson's Disease.
Abstract
Abstract
The diagnosis of Parkinson's disease (PD) is initiated after the occurrence of motor symptoms, such as resting tremors, rigidity, and bradykinesia. According to previous reports, non-motor symptoms, notably gastrointestinal dysfunction, could potentially be early biomarkers in PD patients as such symptoms occur earlier than motor symptoms. However, connecting PD to the intestine is methodologically challenging. Thus, we generated in vitro human intestinal organoids from PD patients and ex vivo mouse small intestinal organoids from aged transgenic mice. Both intestinal organoids (IOs) contained the human LRRK2 G2019S mutation, which is the most frequent genetic cause of familial and sporadic PD. By conducting comprehensive genomic comparisons with these two types of IOs, we determined that a particular gene, namely, Iroquois homeobox protein 2 (IRX2), showed PD-related expression patterns not only in human pluripotent stem cell (PSC)-derived neuroectodermal spheres but also in human PSC-derived neuronal cells containing dopaminergic neurons. We expected that our approach of using various cell types presented a novel technical method for studying the effects of multi-organs in PD pathophysiology as well as for the development of diagnostic markers for PD.
Leukemia 2020 may
CRL3-SPOP ubiquitin ligase complex suppresses the growth of diffuse large B-cell lymphoma by negatively regulating the MyD88/NF-$\kappa$B signaling.
Abstract
Abstract
Recurrent oncogenic mutations of MyD88 have been identified in a variety of lymphoid malignancies. Gain-of-function mutations of MyD88 constitutively activate downstream NF-$\kappa$B signaling pathways, resulting in increased cellular proliferation and survival. However, whether MyD88 activity can be aberrantly regulated in MyD88-wild-type lymphoid malignancies remains poorly understood. SPOP is an adaptor protein of CUL3-based E3 ubiquitin ligase complex and frequently mutated genes in prostate and endometrial cancers. In this study, we reveal that SPOP binds to and induces the nondegradative ubiquitination of MyD88 by recognizing an atypical SPOP-binding motif in MyD88. This modification blocks Myddosome assembly and downstream NF-$\kappa$B activation. SPOP is mutated in a subset of lymphoid malignancies, including diffuse large B-cell lymphoma (DLBCL). Lymphoid malignancies-associated SPOP mutants exhibited impaired binding to MyD88 and suppression of NF-$\kappa$B activation. The DLBCL-associated, SPOP-binding defective mutants of MyD88 escaped from SPOP-mediated ubiquitination, and their effect on NF-$\kappa$B activation is stronger than that of wild-type MyD88. Moreover, SPOP suppresses DLBCL cell growth in vitro and tumor xenograft in vivo by inhibiting the MyD88/NF-$\kappa$B signaling. Therefore, SPOP acts as a tumor suppressor in DLBCL. Mutations in the SPOP-MyD88 binding interface may disrupt the SPOP-MyD88 regulatory axis and promote aberrant MyD88/NF-$\kappa$B activation and cell growth in DLCBL.
Cancers 2020 mar
Hippo-YAP1 Is a Prognosis Marker and Potentially Targetable Pathway in Advanced Gallbladder Cancer.
Abstract
Abstract
Gallbladder cancer is an aggressive disease with late diagnosis and no efficacious treatment. The Hippo-Yes-associated protein 1 (YAP1) signaling pathway has emerged as a target for the development of new therapeutic interventions in cancers. However, the role of the Hippo-targeted therapy has not been addressed in advanced gallbladder cancer (GBC). This study aimed to evaluate the expression of the major Hippo pathway components mammalian Ste20-like protein kinase 1 (MST1), YAP1 and transcriptional coactivator with PDZ-binding motif (TAZ) and examined the effects of Verteporfin (VP), a small molecular inhibitor of YAP1-TEA domain transcription factor (TEAD) protein interaction, in metastatic GBC cell lines and patient-derived organoids (PDOs). Immunohistochemical analysis revealed that advanced GBC patients had high nuclear expression of YAP1. High nuclear expression of YAP1 was associated with poor survival in GBC patients with subserosal invasion (pT2). Additionally, advanced GBC cases showed reduced expression of MST1 compared to chronic cholecystitis. Both VP treatment and YAP1 siRNA inhibited the migration ability in GBC cell lines. Interestingly, gemcitabine resistant PDOs with high nuclear expression of YAP1 were sensitive to VP treatment. Taken together, our results suggest that key components of the Hippo-YAP1 signaling pathway are dysregulated in advanced gallbladder cancer and reveal that the inhibition YAP1 may be a candidate for targeted therapy.
JCI insight 2020 jun
Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer.
Abstract
Abstract
Interferon regulatory factor 1 (IRF1) regulates diverse biological functions, including modulation of cellular responses involved in tumorigenesis. Genetic mutations and altered IRF1 function are associated with several cancers. Although the function of IRF1 in the immunobiology of cancer is emerging, IRF1-specific mechanisms regulating tumorigenesis and tissue homeostasis in vivo are not clear. Here, we found that mice lacking IRF1 were hypersusceptible to colorectal tumorigenesis. IRF1 functions in both the myeloid and epithelial compartments to confer protection against AOM/DSS-induced colorectal tumorigenesis. We further found that IRF1 also prevents tumorigenesis in a spontaneous mouse model of colorectal cancer. The attenuated cell death in the colons of Irf1-/- mice was due to defective pyroptosis, apoptosis, and necroptosis (PANoptosis). IRF1 does not regulate inflammation and the inflammasome in the colon. Overall, our study identified IRF1 as an upstream regulator of PANoptosis to induce cell death during colitis-associated tumorigenesis.
Cellular microbiology 2020 jun
Germ-free and microbiota-associated mice yield small intestinal epithelial organoids with equivalent and robust transcriptome/proteome expression phenotypes.
Abstract
Abstract
Intestinal epithelial organoids established from gut tissue have become a widely used research tool. However, it remains unclear how environmental cues, divergent microbiota composition and other sources of variation before, during and after establishment confound organoid properties, and how these properties relate to the original tissue. While environmental influences cannot be easily addressed in human organoids, mice offer a controlled assay-system. Here, we probed the effect of donor microbiota differences, previously identified as a confounding factor in murine in vivo studies, on organoids. We analysed the proteomes and transcriptomes of primary organoid cultures established from two colonised and one germ-free mouse colony of C57BL/6J genetic background, and compared them to their tissue of origin and commonly used cell lines. While an imprint of microbiota-exposure was observed on the proteome of epithelial samples, the long-term global impact of donor microbiota on organoid expression patterns was negligible. Instead, stochastic culture-to-culture differences accounted for a moderate variability between independently established organoids. Integration of transcriptome and proteome datasets revealed an organoid-typic expression signature comprising 14 transcripts and 10 proteins that distinguished organoids across all donors from murine epithelial cell lines and fibroblasts and closely mimicked expression patterns in the gut epithelium. This included the inflammasome components ASC, Naip1-6, Nlrc4 and Caspase-1, which were highly expressed in all organoids compared to the reference cell line m-ICc12 or mouse embryonic fibroblasts. Taken together, these results reveal that the donor microbiota has little effect on the organoid phenotype and suggest that organoids represent a more suitable culture model than immortalised cell lines, in particular for studies of intestinal epithelial inflammasomes.