MethoCult™ H4034 Optimum
Methylcellulose-based medium with recombinant cytokines for human cells
概要
MethoCult™ H4034 Optimum (MethoCult™ GF H4034) is a methylcellulose-based medium for the growth and enumeration of hematopoietic progenitor cells in colony-forming unit (CFU) assays of human bone marrow, mobilized peripheral blood, peripheral blood, and cord blood samples. MethoCult™ H4034 Optimum has been formulated to support optimal growth of erythroid progenitor cells (BFU-E and CFU-E), granulocyte-macrophage progenitor cells (CFU-GM, CFU-G and CFU-M), and multipotential granulocyte, erythroid, macrophage, megakaryocyte progenitor cells (CFU-GEMM). The inclusion of G-CSF in Optimum formulations improves discrimination of myeloid colony subtypes. This formulation is compatible with STEMvision™ software for automated colony counting.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
Browse our Frequently Asked Questions (FAQs) on performing the CFU assay and explore its utility as part of the cell therapy workflow.
Contains
• Methylcellulose in Iscove's MDM
• Fetal bovine serum
• Bovine serum albumin
• 2-Mercaptoethanol
• Recombinant human stem cell factor (SCF)
• Recombinant human interleukin 3 (IL-3)
• Recombinant human erythropoietin (EPO)
• Recombinant human granulocyte colony-stimulating factor (G-CSF)
• Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF)
• Supplements
• Fetal bovine serum
• Bovine serum albumin
• 2-Mercaptoethanol
• Recombinant human stem cell factor (SCF)
• Recombinant human interleukin 3 (IL-3)
• Recombinant human erythropoietin (EPO)
• Recombinant human granulocyte colony-stimulating factor (G-CSF)
• Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF)
• Supplements
Subtype
Semi-Solid Media, Specialized Media
Cell Type
Hematopoietic Stem and Progenitor Cells
Species
Human, Non-Human Primate
Application
Cell Culture, Colony Assay, Functional Assay
Brand
MethoCult
Area of Interest
Drug Discovery and Toxicity Testing, Stem Cell Biology
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | MethoCult™ H4034 Optimum | 04034, 04044 | All | English |
| Manual | MethoCult™ H4034 Optimum | 04034 | All | English |
| Safety Data Sheet | MethoCult™ H4034 Optimum | 04034, 04044 | All | English |
数据及文献
Data
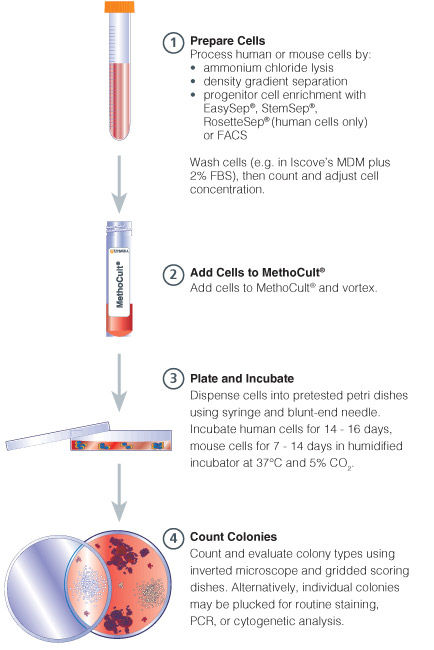
Figure 1. Procedure Summary for Hematopoietic CFU Assays
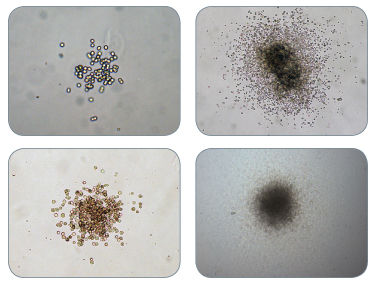
Figure 2. Examples of Colonies Derived from CFU-GM in MethoCult™ H4034 Optimum
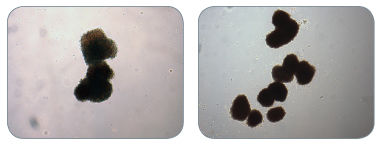
Figure 3. Examples of Colonies Derived from BFU-E in MethoCult™ H4034 Optimum
Publications (23)
Cell stem cell 2019 mar
The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance.
Abstract
Abstract
It has been recently shown that increased oxidative phosphorylation, as reflected by increased mitochondrial activity, together with impairment of the mitochondrial stress response, can severely compromise hematopoietic stem cell (HSC) regeneration. Here we show that the NAD+-boosting agent nicotinamide riboside (NR) reduces mitochondrial activity within HSCs through increased mitochondrial clearance, leading to increased asymmetric HSC divisions. NR dietary supplementation results in a significantly enlarged pool of progenitors, without concurrent HSC exhaustion, improves survival by 80{\%}, and accelerates blood recovery after murine lethal irradiation and limiting-HSC transplantation. In immune-deficient mice, NR increased the production of human leucocytes from hCD34+ progenitors. Our work demonstrates for the first time a positive effect of NAD+-boosting strategies on the most primitive blood stem cells, establishing a link between HSC mitochondrial stress, mitophagy, and stem-cell fate decision, and unveiling the potential of NR to improve recovery of patients suffering from hematological failure including post chemo- and radiotherapy.
Nature communications 2019
Gene correction for SCID-X1 in long-term hematopoietic stem cells.
Abstract
Abstract
Gene correction in human long-term hematopoietic stem cells (LT-HSCs) could be an effective therapy for monogenic diseases of the blood and immune system. Here we describe an approach for X-linked sSevere cCombined iImmunodeficiency (SCID-X1) using targeted integration of a cDNA into the endogenous start codon to functionally correct disease-causing mutations throughout the gene. Using a CRISPR-Cas9/AAV6 based strategy, we achieve up to 20{\%} targeted integration frequencies in LT-HSCs. As measures of the lack of toxicity we observe no evidence of abnormal hematopoiesis following transplantation and no evidence of off-target mutations using a high-fidelity Cas9 as a ribonucleoprotein complex. We achieve high levels of targeting frequencies (median 45{\%}) in CD34+ HSPCs from six SCID-X1 patients and demonstrate rescue of lymphopoietic defect in a patient derived HSPC population in vitro and in vivo. In sum, our study provides specificity, toxicity and efficacy data supportive of clinical development of genome editing to treat SCID-Xl.
Blood 2017 MAR
Identification of unipotent megakaryocyte progenitors in human hematopoiesis.
Abstract
Abstract
The developmental pathway for human megakaryocytes remains unclear and the definition of pure unipotent megakaryocyte progenitor is still controversial. Using single-cell transcriptome analysis, we have identified a cluster of cells within immature hematopoietic stem and progenitor cell populations that specifically express genes related to the megakaryocyte lineage. We used CD41 as a positive marker to identify these cells within the CD34(+)CD38(+)IL-3Rα(dim)CD45RA(-) common myeloid progenitor (CMP) population. These cells lacked erythroid and granulocyte/macrophage potential, but exhibited robust differentiation into the megakaryocyte lineage at a high frequency, both in vivo and in vitro The efficiency and expansion potential of these cells exceeded those of conventional bipotent megakaryocyte/erythrocyte progenitors. Accordingly, the CD41(+) CMP was defined as a unipotent megakaryocyte progenitor (MegP) that is likely to represent the major pathway for human megakaryopoiesis, independent of canonical megakaryocyte-erythroid lineage bifurcation. In the bone marrow of patients with essential thrombocythemia, the MegP population was significantly expanded in the context of a high burden of Janus kinase 2 mutations. Thus, the prospectively isolatable and functionally homogeneous human MegP will be useful for the elucidation of the mechanisms underlying normal and malignant human hematopoiesis.
Nature 2016 NOV
CRISPR/Cas9 $\beta$-globin gene targeting in human haematopoietic stem cells.
Abstract
Abstract
The $\beta$-haemoglobinopathies, such as sickle cell disease and $\beta$-thalassaemia, are caused by mutations in the $\beta$-globin (HBB) gene and affect millions of people worldwide. Ex vivo gene correction in patient-derived haematopoietic stem cells followed by autologous transplantation could be used to cure $\beta$-haemoglobinopathies. Here we present a CRISPR/Cas9 gene-editing system that combines Cas9 ribonucleoproteins and adeno-associated viral vector delivery of a homologous donor to achieve homologous recombination at the HBB gene in haematopoietic stem cells. Notably, we devise an enrichment model to purify a population of haematopoietic stem and progenitor cells with more than 90{\%} targeted integration. We also show efficient correction of the Glu6Val mutation responsible for sickle cell disease by using patient-derived stem and progenitor cells that, after differentiation into erythrocytes, express adult $\beta$-globin (HbA) messenger RNA, which confirms intact transcriptional regulation of edited HBB alleles. Collectively, these preclinical studies outline a CRISPR-based methodology for targeting haematopoietic stem cells by homologous recombination at the HBB locus to advance the development of next-generation therapies for $\beta$-haemoglobinopathies.
Exp Mol Med 2016 MAY
Generation and characterization of integration-free induced pluripotent stem cells from patients with autoimmune disease
Abstract
Abstract
Autoimmune diseases (AIDs), a heterogeneous group of immune-mediated disorders, are a major and growing health problem. Although AIDs are currently treated primarily with anti-inflammatory and immunosuppressive drugs, the use of stem cell transplantation in patients with AIDs is becoming increasingly common. However, stem cell transplantation therapy has limitations, including a shortage of available stem cells and immune rejection of cells from nonautologous sources. Induced pluripotent stem cell (iPSC) technology, which allows the generation of patient-specific pluripotent stem cells, could offer an alternative source for clinical applications of stem cell therapies in AID patients. We used nonintegrating oriP/EBNA-1-based episomal vectors to reprogram dermal fibroblasts from patients with AIDs such as ankylosing spondylitis (AS), Sjogren's syndrome (SS) and systemic lupus erythematosus (SLE). The pluripotency and multilineage differentiation capacity of each patient-specific iPSC line was validated. The safety of these iPSCs for use in stem cell transplantation is indicated by the fact that all AID-specific iPSCs are integrated transgene free. Finally, all AID-specific iPSCs derived in this study could be differentiated into cells of hematopoietic and mesenchymal lineages in vitro as shown by flow cytometric analysis and induction of terminal differentiation potential. Our results demonstrate the successful generation of integration-free iPSCs from patients with AS, SS and SLE. These findings support the possibility of using iPSC technology in autologous and allogeneic cell replacement therapy for various AIDs, including AS, SS and SLE.
Experimental hematology 2016 JAN
GSK3$\$ activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells.
Abstract
Abstract
WNT/$\$-CATENIN signaling promotes the hematopoietic/endothelial differentiation of human embryonic stem cells and human induced pluripotent stem cells (hiPSCs). The transient addition of a GSK3$\$ (GSKi) has been found to facilitate in vitro endothelial cell differentiation from hESCs/hiPSCs. Because hematopoietic and endothelial cells are derived from common progenitors (hemogenic endothelial progenitors [HEPs]), we examined the effect of transient GSKi treatment on hematopoietic cell differentiation from hiPSCs. We found that transient GSKi treatment at the start of hiPSC differentiation induction altered the gene expression profile of the cells. Multiple CDX/HOX genes, which are expressed in the posterior mesoderm of developing embryos, were significantly upregulated by GSKi treatment. Further, inclusion of the GSKi in a serum- and stroma-free culture with chemically defined medium efficiently induced HEPs, and the HEPs gave rise to various lineages of hematopoietic and endothelial cells. Therefore, transient WNT/$\$-CATENIN signaling triggers activation of the CDX/HOX pathway, which in turn confers hemogenic posterior mesoderm identity to differentiating hiPSCs. These data enhance our understanding of human embryonic hematopoietic/endothelial cell development and provide a novel in vitro system for inducing the differentiation of hematopoietic cells from hiPSCs.