EC-Cult™-XF Culture Kit
EC-Cult™-XF Medium must be used in conjunction with Animal Component-Free Cell Attachment Substrate (Component #07130; included in EC-Cult™-XF Culture Kit). For passaging, Animal Component-Free Cell Dissociation Kit (Catalog #05426) is required. EC-Cult™-XF Medium must be supplemented with Heparin Solution (Catalog #07980). Since Heparin Solution contains non-human animal-derived components, the complete medium will not be xeno-free.
• Serum-free formulation supports the derivation, expansion, and maintenance of ECFCs
• Rigorous raw material screening and quality control minimize lot-to-lot variability and increase reproducibility between experiments
- EC-Cult™-XF Basal Medium, 150 mL
- EC-Cult™-XF 2.5X Supplement, 100 mL
- Animal Component-Free Cell Attachment Substrate, 1 mL
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EC-Cult™-XF Culture Kit | 08000 | All | English |
| Manual | EC-Cult™-XF Culture Kit | 08000 | All | English |
| Safety Data Sheet 1 | EC-Cult™-XF Culture Kit | 08000 | All | English |
| Safety Data Sheet 2 | EC-Cult™-XF Culture Kit | 08000 | All | English |
| Safety Data Sheet 3 | EC-Cult™-XF Culture Kit | 08000 | All | English |
Data
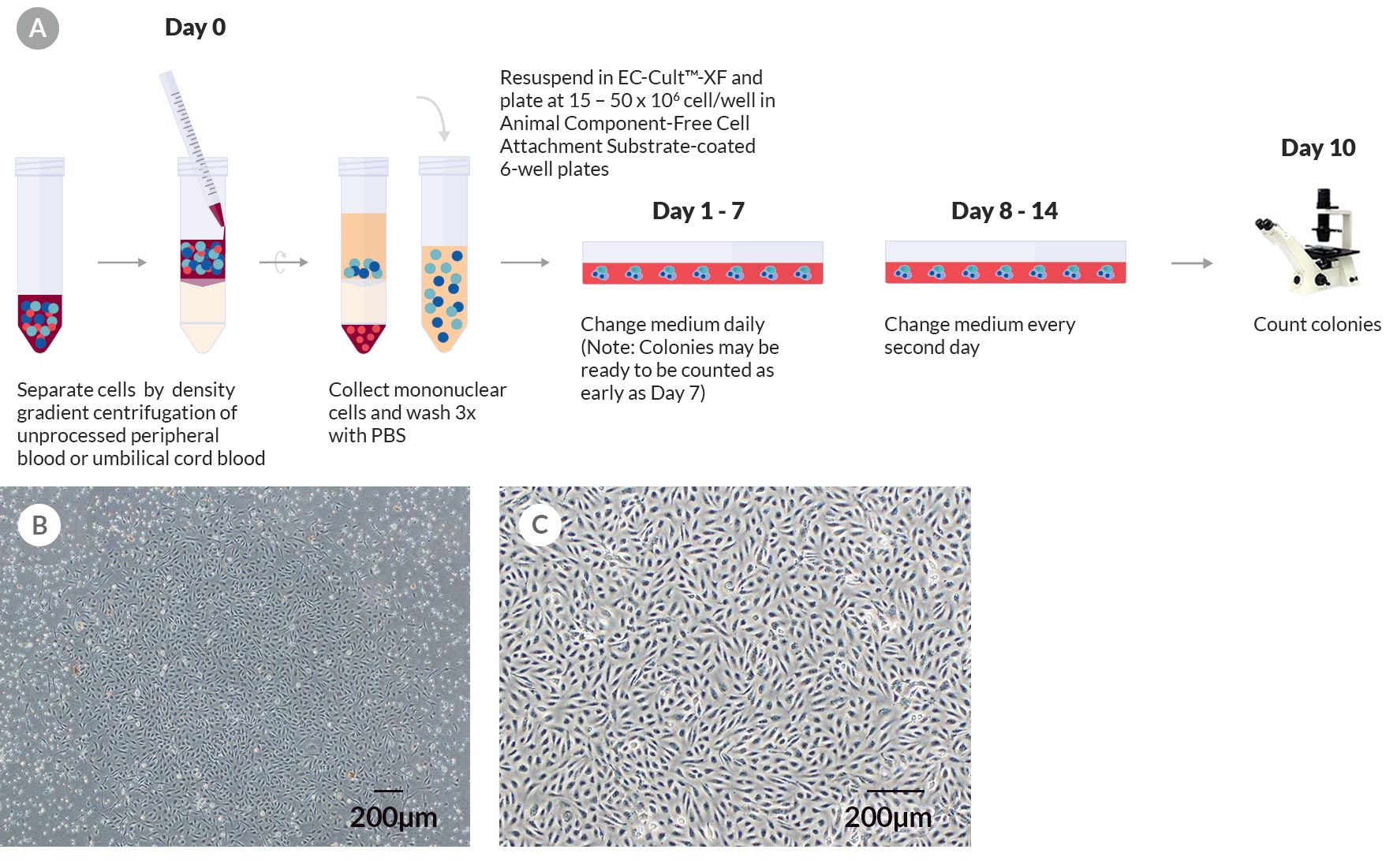
Figure 1. Derivation and Colony Formation Assay of Human ECFCs Using EC-Cult™-XF Culture Kit
(A) A schematic of the colony formation assay for enumerating ECFC colonies derived in EC-Cult™-XF Culture Medium. On day 0, mononuclear cells are isolated from whole blood and plated on Animal Component-Free Cell Attachment Substrate-coated plates in EC-Cult™-XF Culture Medium. From day 1 to 7, nonadherent cells are gently removed through daily medium changes. From day 8 onwards, the medium is changed every second day until cells are ready to be passaged. Colonies should be enumerated around day 10 before they start merging. (B) An umbilical cord blood-derived ECFC colony on day 11. (C) A representative phase-contrast image at passage 3 of ECFCs derived and expanded in complete EC-Cult™-XF ECFC Medium. These cells grow as monolayers and retain their typical cobblestone-like morphology of endothelial cells.
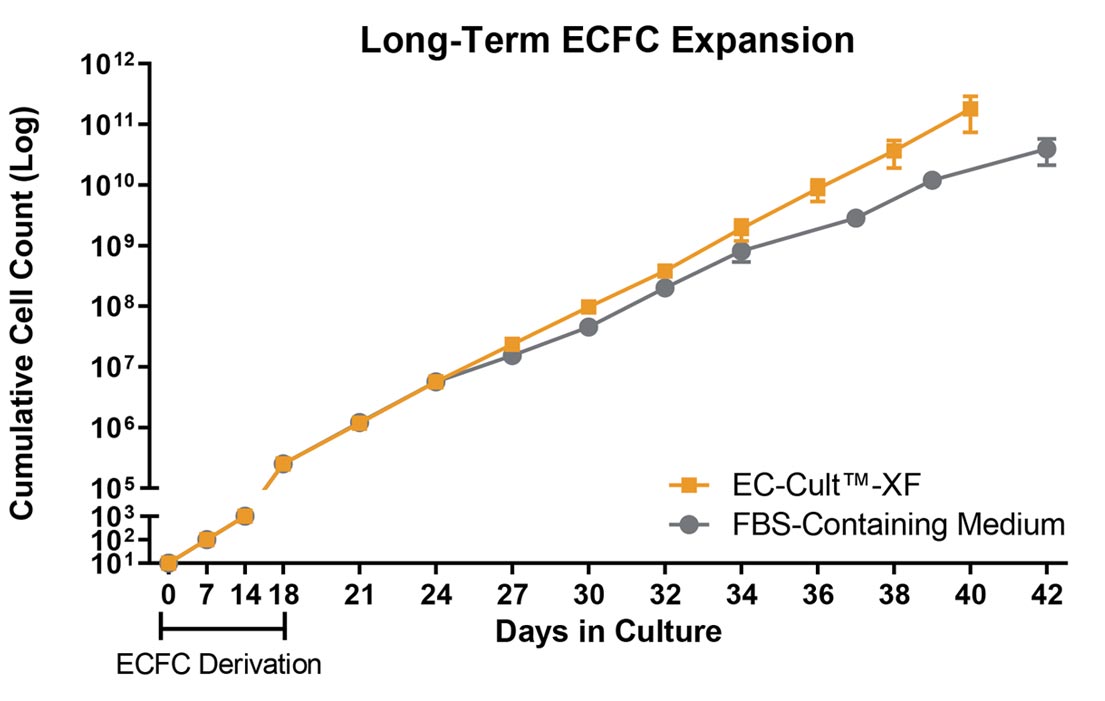
Figure 2. Human ECFCs Expand More Rapidly When Using EC-Cult™-XF Culture Kit
Human cord blood-derived ECFCs exhibit a greater expansion rate in complete EC-Cult™-XF Culture Medium than in FBS-containing commercial medium. When cultured in EC-Cult™-XF, cells retain their rate of expansion in later passages, while the rate of expansion in the commercial medium declines as early as passage 6. Error bars represent standard error of mean (SEM; n = 4).
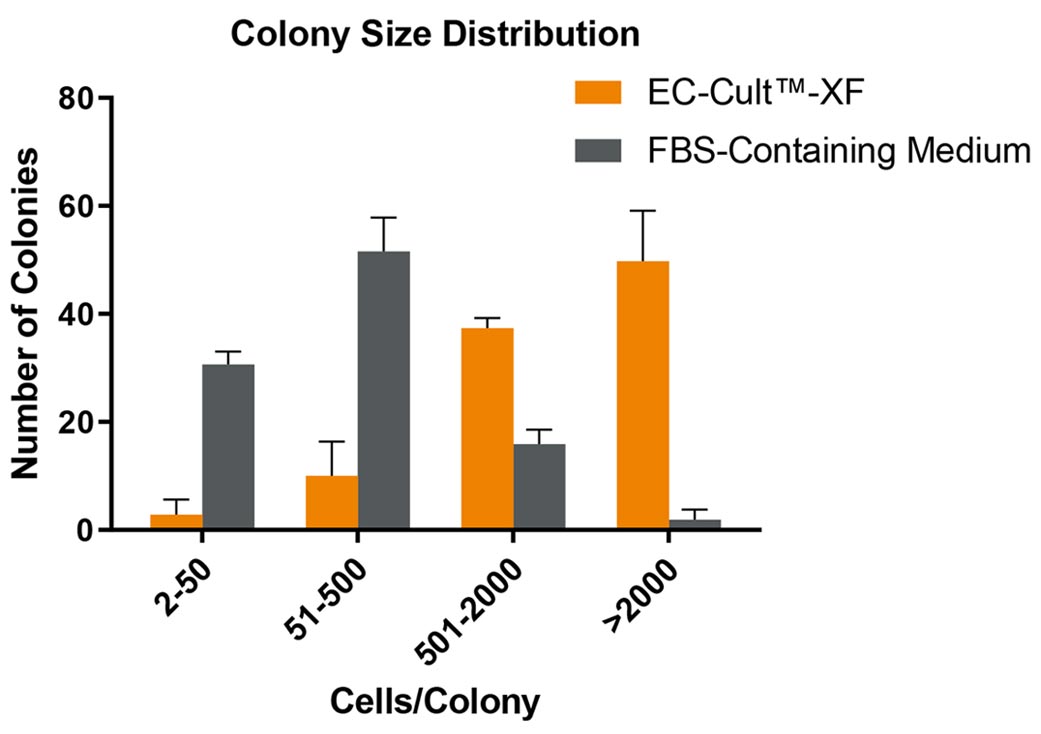
Figure 3. ECFCs Derived and Expanded in EC-Cult™-XF Culture Medium Maintain High Proliferative Clonogenic Potential
Human cord blood-derived ECFCs were plated in complete EC-Cult™-XF Culture Medium or an FBS-containing commercial medium at single cell per well density and were monitored for colony formation over a 14-day period. Assessment of colony formation and size is used to determine a cell’s hierarchical proliferative potential. This assessment includes counting and classifying the number of cells per colony as endothelial clusters (2 - 50 cells/colony), low proliferative (51 - 500 cells/colony), medium proliferative (501 - 2000 cells/colony), and high proliferative (>2000 cells/colony) ECFCs. 40 - 60% of ECFCs formed well-circumscribed colonies with >2000 cells when cultured in EC-Cult™-XF, in contrast to only about 2% highly proliferative colonies detected in the FBS-containing commercial medium. Error bars represent SEM (n = 3).
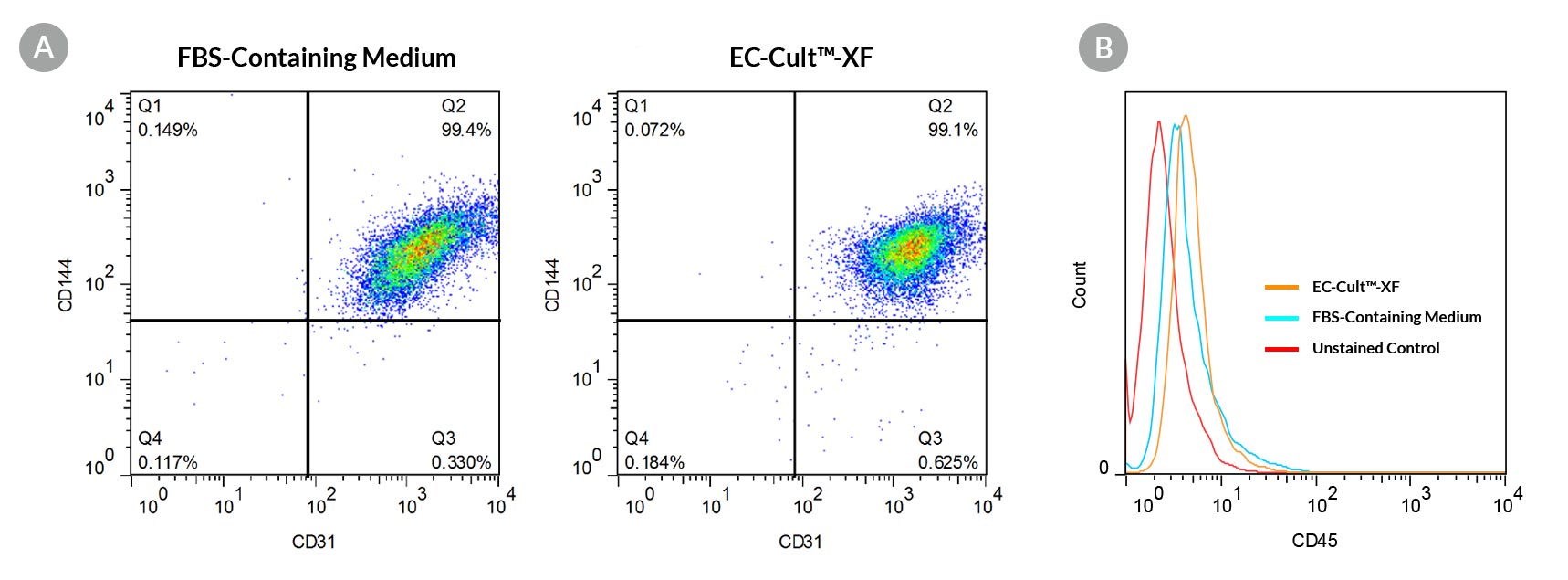
Figure 4. ECFCs Expanded in EC-Cult™-XF Culture Medium Form a Homogeneous Population That Expresses High Levels of Endothelial Cell Surface Markers
Human cord blood ECFCs derived and expanded for 5 passages in EC-Cult™-XF Culture Medium (A) maintain high expression levels of endothelial cell surface markers (CD31 and CD144) and (B) lack the expression of the hematopoietic marker, CD45.
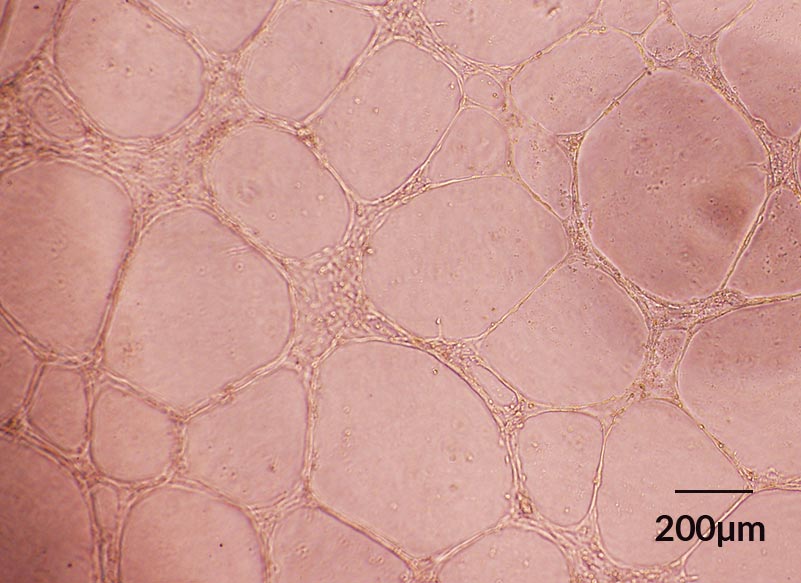
Figure 5. ECFCs Derived in EC-Cult™-XF ECFC Medium Form Angiogenic Tubular Networks In Vitro
Human cord blood ECFCs derived and expanded for 5 passages in EC-CultTM-XF Culture Medium maintain functionality and spontaneously assemble into capillary tube-like structures overnight when seeded on Corning® Matrigel®-coated plates.
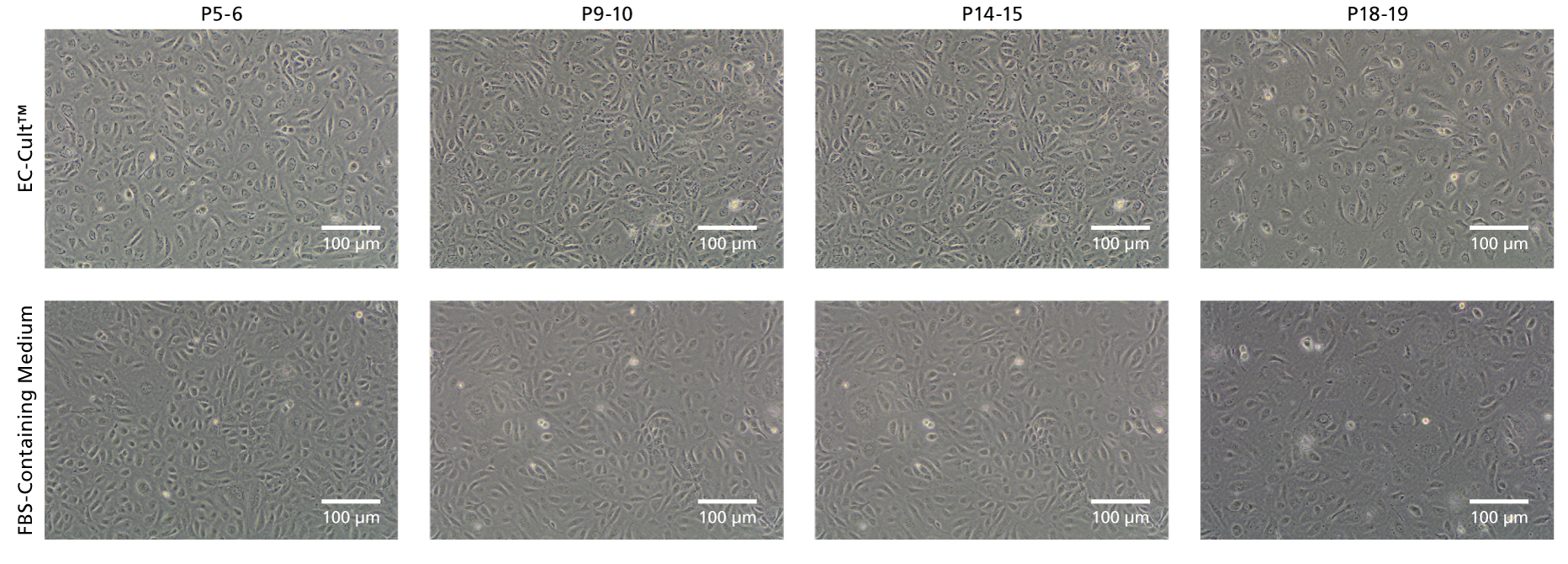
Figure 6. EC-Cult™ Medium Enables Long-Term Expansion of HUVECs in an FBS-Free Setting
Cryopreserved human umbilical vein endothelial cells (HUVECs) were thawed in either EC-Cult™ Medium or a fetal bovine serum (FBS)-containing commercial medium and replated at 1x10^5 cells/well (6-well plate) in the same media. Cells cultured in EC-Cult™ Medium were passaged using the Animal Component-Free Cell Dissociation Kit. Cells cultured in FBS-containing commercial medium were cultured and passaged according to manufacturer’s protocol. HUVECs cultured in EC-Cult™ Medium display the typical cobblestone morphology of endothelial cells.
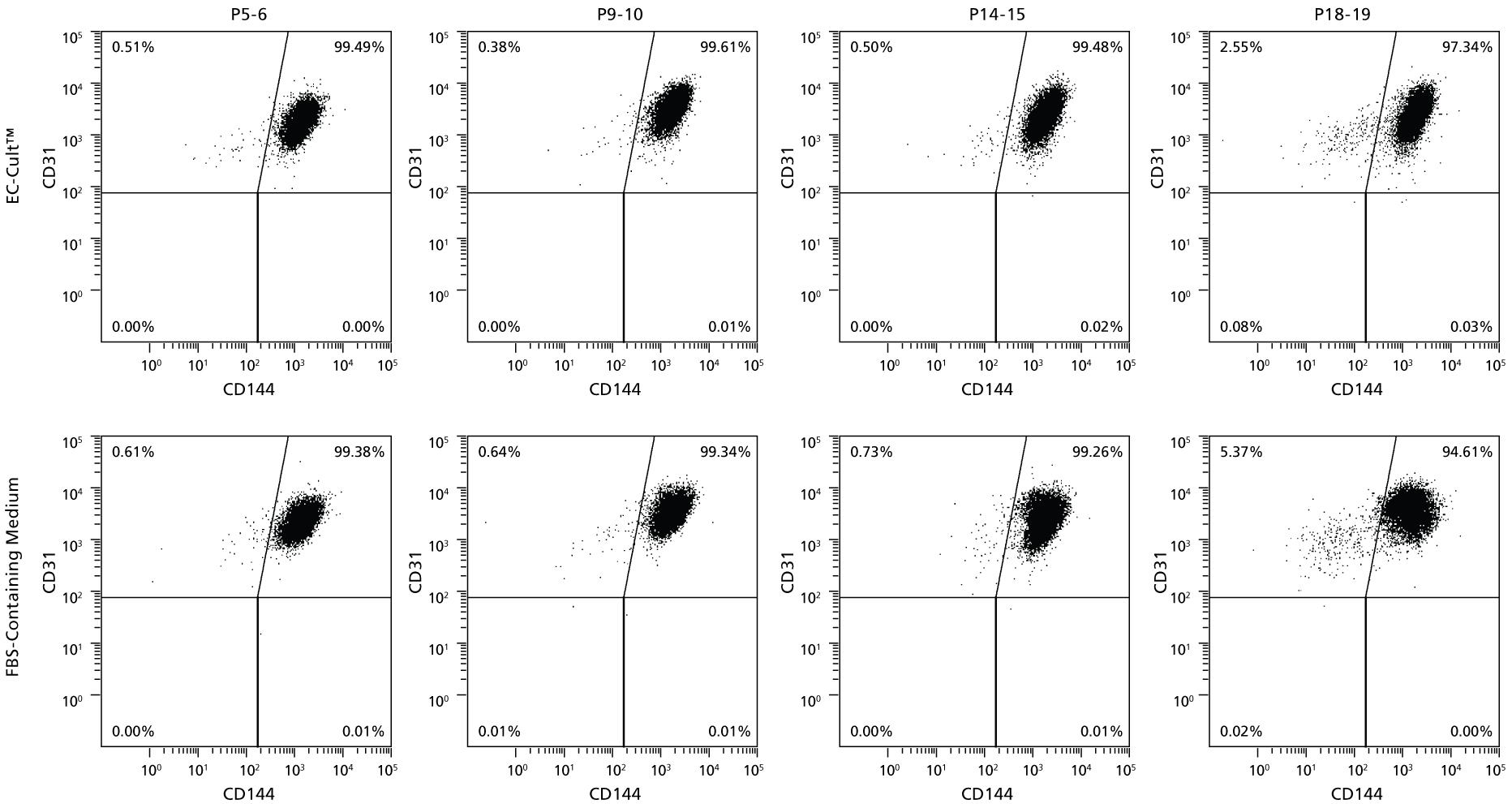
Figure 7. HUVECs Cultured Long-Term in EC-Cult™ Medium Maintain High Levels of Endothelial Markers
Human umbilical vein endothelial cells (HUVECs) cultured and passaged in EC-Cult™ Medium (analyzed by flow cytometry) retained the endothelial markers CD31 and CD144 through 19 passages.
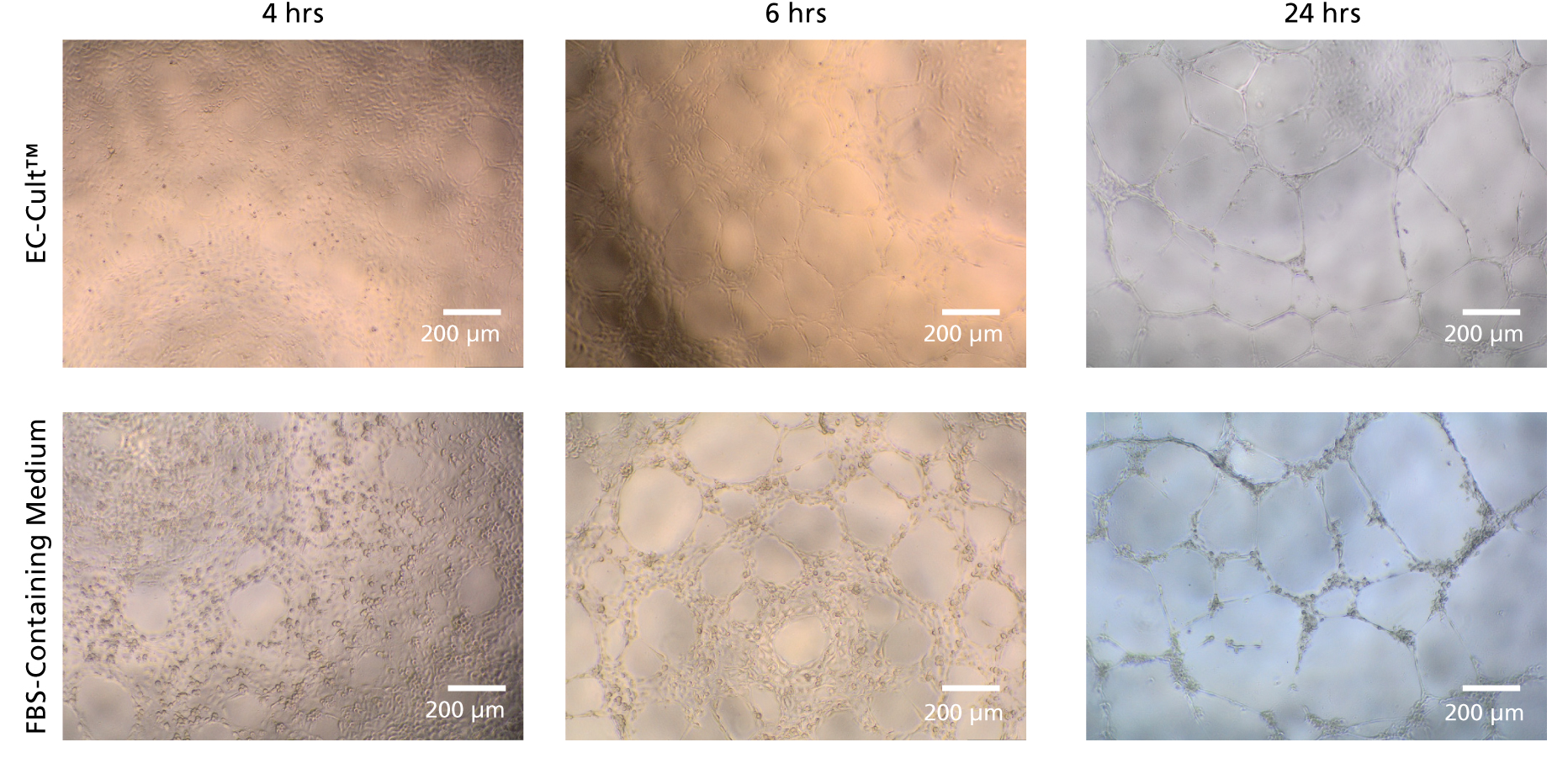
Figure 8. HUVECs Form Tubular Networks When Cultured in EC-Cult™ Medium
Human umbilical vein endothelial cells (HUVECS) were expanded for 18 passages in EC-Cult™ Media or a commercial FBS-containing medium. The cells were then plated at 1.0 x 10^4 cells/well (96-well plate) in EC-Cult™ Medium and grown for 24 hours on Corning® Matrigel®. Network formations were imaged at different time points after plating. HUVECS cultured in EC-Cult™ media formed higher quality, more defined tubular branching points.
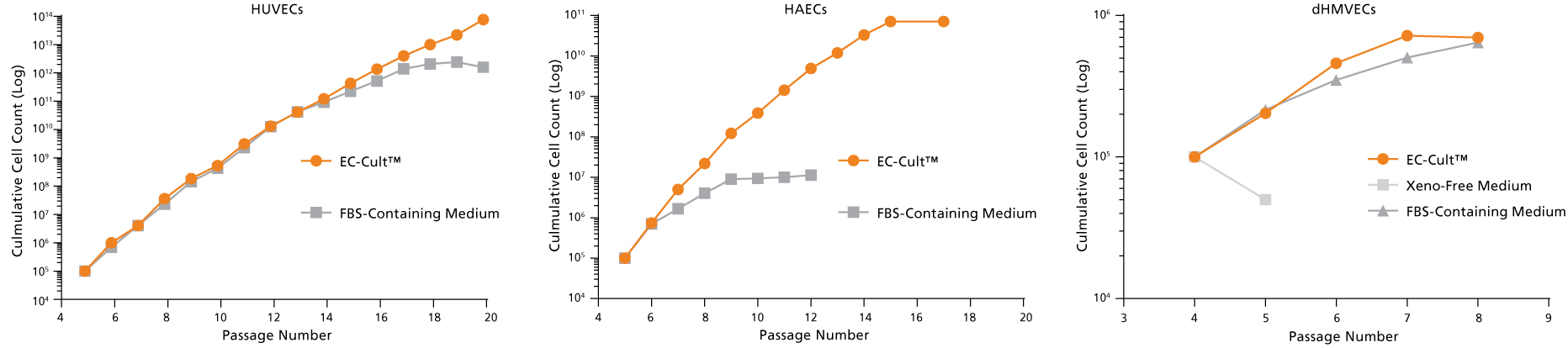
Figure 9. EC-Cult™ Medium Can Be Used to Expand Different Endothelial Cell Types
Cryopreserved human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs), and dermal human microvascular endothelial cells (dHMVECs) were expanded using EC-Cult™ Medium or other commercial media according to their respective manufacturers’ protocols and counted after each passage. Cells expanded more rapidly and for longer passage numbers in EC-Cult™ Medium.