ClonaCell™-HY AOF Expansion Medium
This medium has been verified for use with mouse and rat hybridomas and is suitable for expansion of mouse myelomas such as Sp2/0, rat myelomas such as YB2/0, and human myelomas such as U266. In most cases, cryopreserved hybridomas can be thawed directly into ClonaCell™-HY AOF Expansion Medium while maintaining a high level of viability. This medium may be used as a serum-free alternative to ClonaCell™-HY Medium A (Catalog #03801) or ClonaCell™-HY Medium E (Catalog #03805). No materials of animal or human origin are used in the manufacture of this medium or its components, to at least the secondary level of manufacturing.
• Absence of serum reduces performance variability in the medium
• Simplifies downstream clone screening and antibody purification (no serum-derived IgG)
• Gentamicin
• Phenol red
• L-Glutamine and other supplements
• Other ingredients, including recombinant proteins
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | ClonaCell™HY AOF Expansion Medium | 03835 | All | English |
| Manual | ClonaCell™-HY AOF Expansion Medium | 03835 | All | English |
| Safety Data Sheet | ClonaCell™HY AOF Expansion Medium | 03835 | All | English |
Data
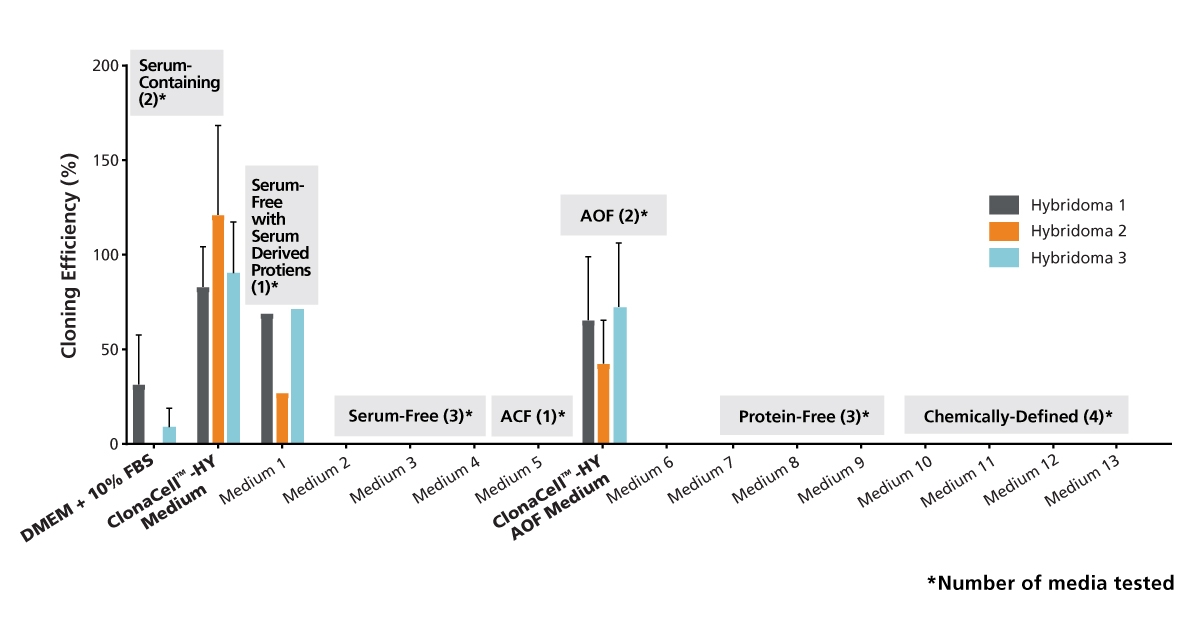
Figure 1. Cloning Efficiencies for Three Hybridoma Cell Lines Subcloned in Serum-containing and Serum-free Cell Culture Media
The hybridoma lines were adapted to growth in each medium and subcloned by limiting dilution (n = 1 - 5). After incubation for 10 days (37°C, 5% CO2), the plates were analyzed with a Cell Metric™ instrument (Solentim). The plating efficiency was estimated by Poisson statistics using the ELDA method described by Hu & Smith, 2009 (J Immunol Meth, 347: 70-78). ACF = animal component-free. Data is expressed as mean + 1 SD.
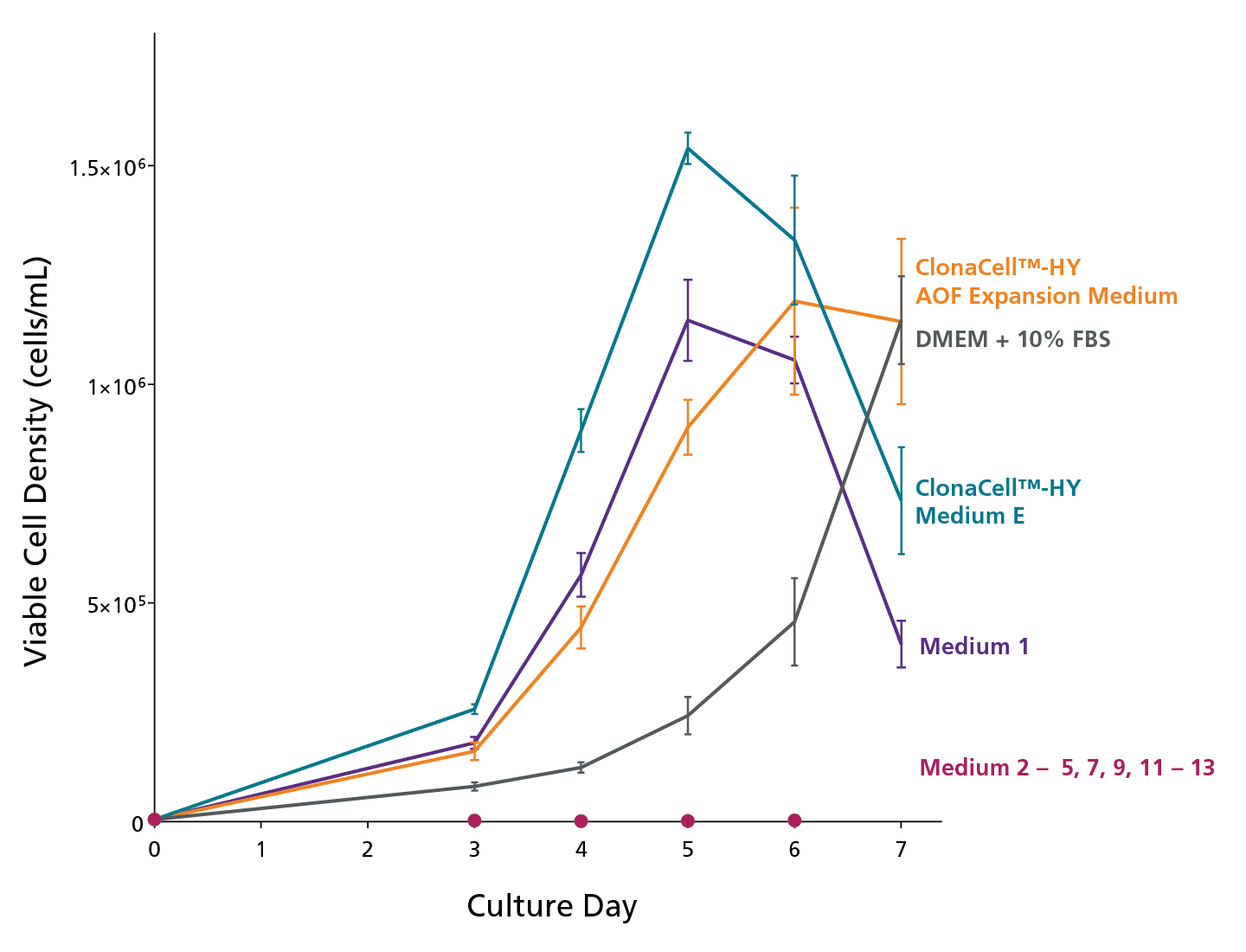
Figure 2. Expansion of an Established Hybridoma Cell Line in Several Commercially-Available Serum-Containing and Serum-Free Hybridoma Cell Culture Media
The hybridoma line was adapted to growth in 13 different cell culture media: ClonaCellTM-HY AOF Expansion Medium (animal origin-free), DMEM + 10% FBS or ClonaCellTM-HY Medium E (serum-containing), Medium 1 (serum-free with serum-derived proteins), Medium 2 – 4 (serum-free), Medium 5 (animal component-free), Medium 7, 9 (protein-free), and Medium 11 – 13 (chemically-defined). They were then seeded in triplicate in 24-well tissue culture plates at 5 x 103 cells/mL. The cultures were incubated at 37°C (5% CO2 atmosphere) and the viable cell density was measured on days 3 – 7. Data is expressed as mean ± 1SD.