BrainPhys™ Imaging Optimized Medium
Applications of BrainPhys™ Imaging Optimized Medium include live fluorescent imaging (calcium imaging and optogenetics) and neuronal culture. In addition to the removal of phenol red, the formulation has been modified to reduce background fluorescence and increase stability upon repeated exposure to light (M Zabolocki et al. Nat Comm, 2020).
• Reduced background autofluorescence in the 488 nm excitation channel
• Perform functional assays without changing media and shocking cells
• More representative of the brain's extracellular environment
• Improved neuronal function and a higher proportion of synaptically active neurons
• Rigorous raw material screening and quality control ensure minimal lot-to-lot variability
- BrainPhys™ Imaging Optimized Medium (Catalog #05796)
- BrainPhys™ Imaging Optimized, 500 mL
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | BrainPhys™ Imaging Optimized Medium | 05796 | All | English |
| Safety Data Sheet | BrainPhys™ Imaging Optimized Medium | 05796 | All | English |
Data
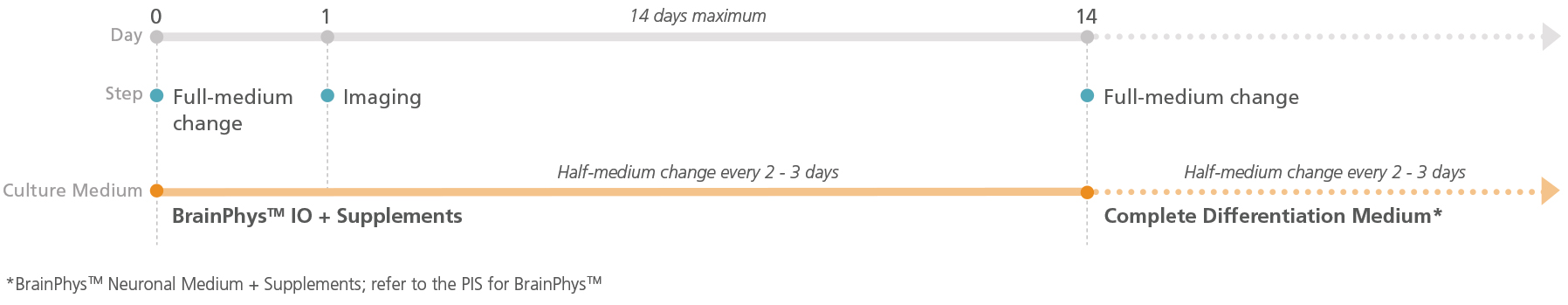
Figure 1. Schematic for Live Imaging During Differentiation of hPSC-Derived Neurons
Neurons can be transitioned into BrainPhys™ IO with the relevant supplements and cultured for a maximum of 14 days.
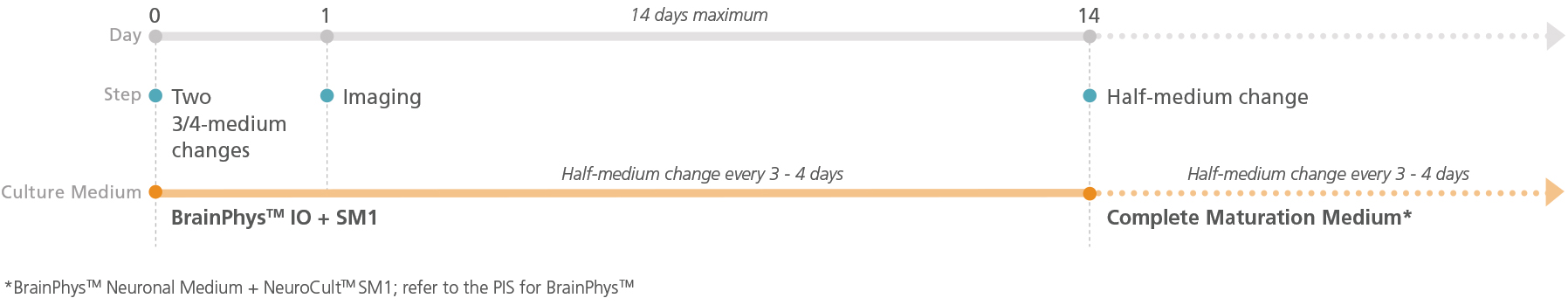
Figure 2. Schematic for Live Imaging During Maturation of Primary Tissue-Derived Neurons
Neurons can be transitioned into BrainPhys™ IO with serum replacement supplement and cultured for a maximum of 14 days.
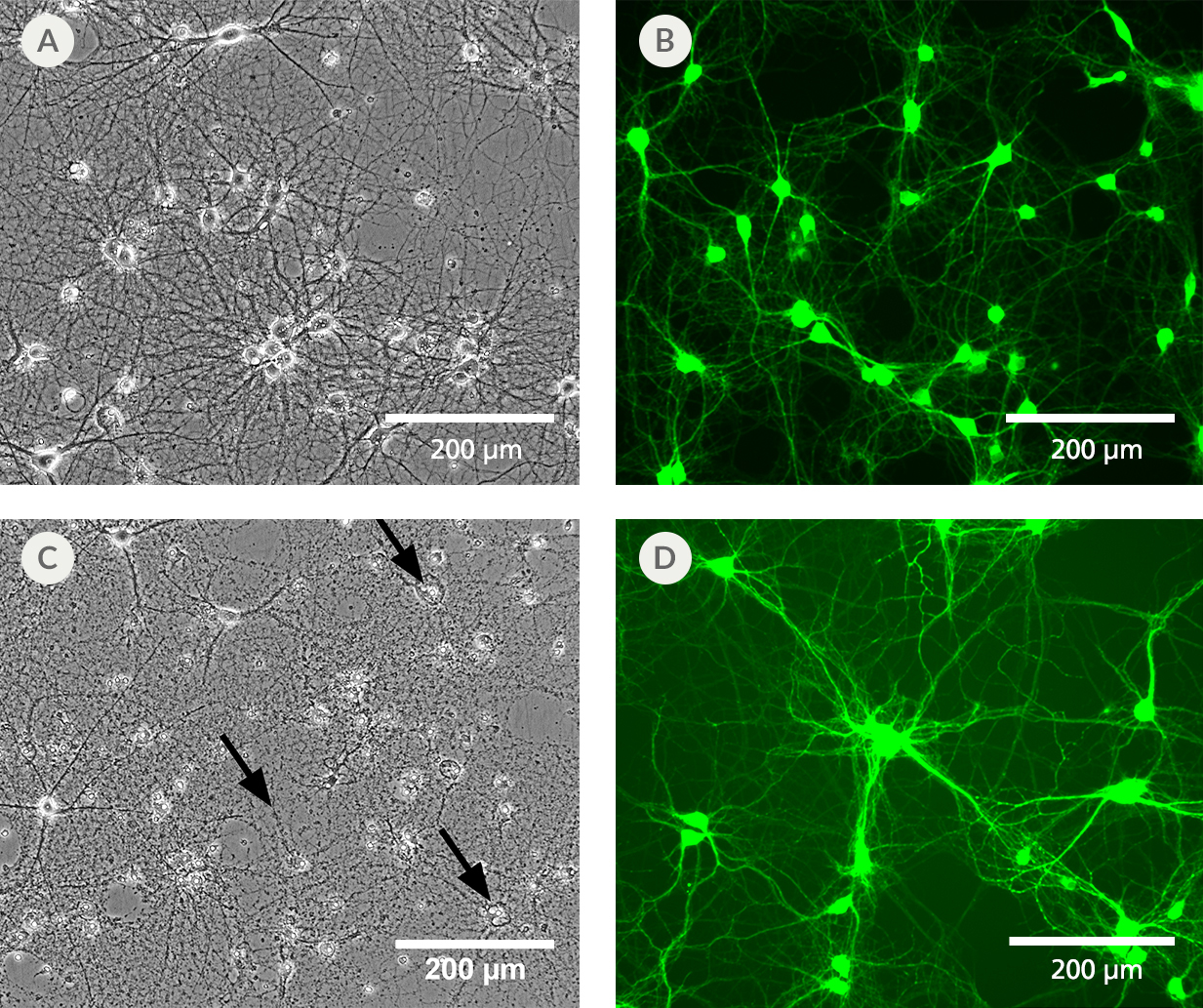
Figure 3. BrainPhys™ IO Reduces Phototoxicity and Autofluorescence of Imaged Cells
Primary rat cortical neurons cultured in BrainPhys™ Imaging Optimized Medium (A) retain a healthy morphology after exposure to blue LED light for 12 hours. (B) Neurons labeled with live neuron dye NeuroFluor™ NeuO showed reduced background fluorescence at a mean emission of 525 nm, resulting in improved image contrast. This medium has enhanced performance under live imaging conditions compared to the original BrainPhys™ Neuronal Medium formulation, which provides superior long-term culture health, but shows (C) some disintegrated cell bodies and neurites (black arrows) and (D) autofluorescence under the same experimental conditions.
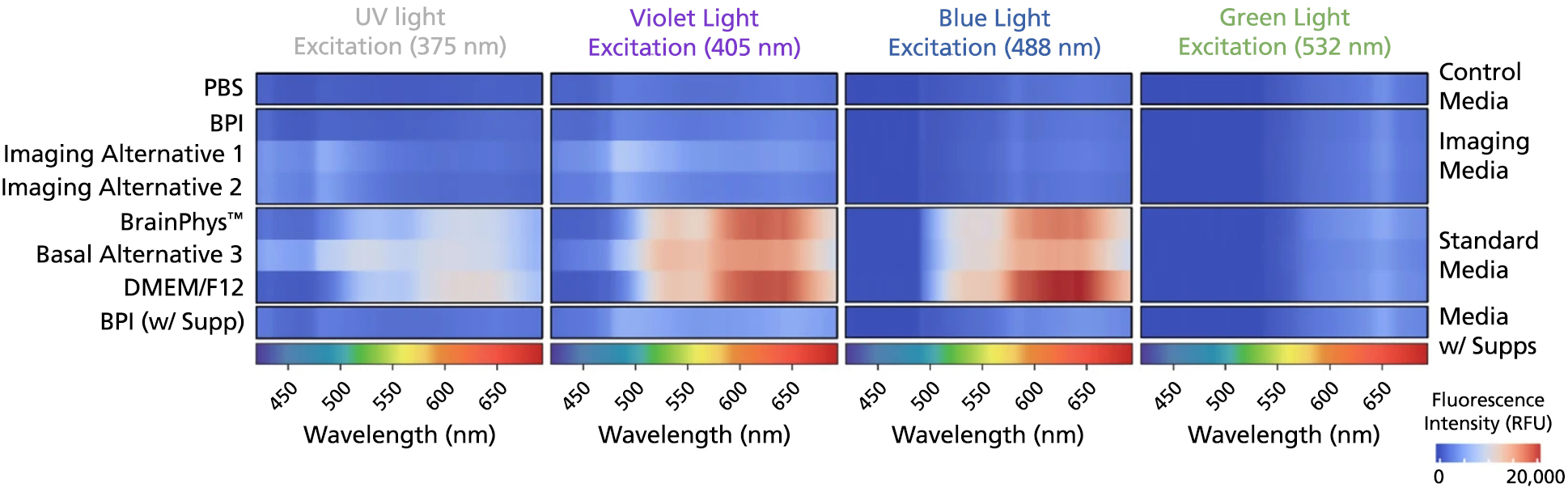
Figure 4. BrainPhys™ Imaging Optimized Medium Reduces Autofluorescence Relative to Standard Neural Media and Other Imaging-Specialized Media
The emission spectra across 400 - 700 nm of the light spectrum were captured as mean autofluorescence intensity from test and control (PBS) media (without cells) for the 375 (ultraviolet), 405 (violet), 488 (blue), and 532 nm (green) excitation wavelengths. Mean fluorescence intensity in PBS was subtracted from other media to normalize the data (n = 8 replicate wells, 3 independent experiments). BrainPhys™ Imaging Optimized (BPI) shows autofluorescence intensities similar to PBS and far lower intensities than are observed from standard neural media. Adapted from Zabolocki et al. 2020, Nature Communications, available under a Creative Commons 4.0 License.
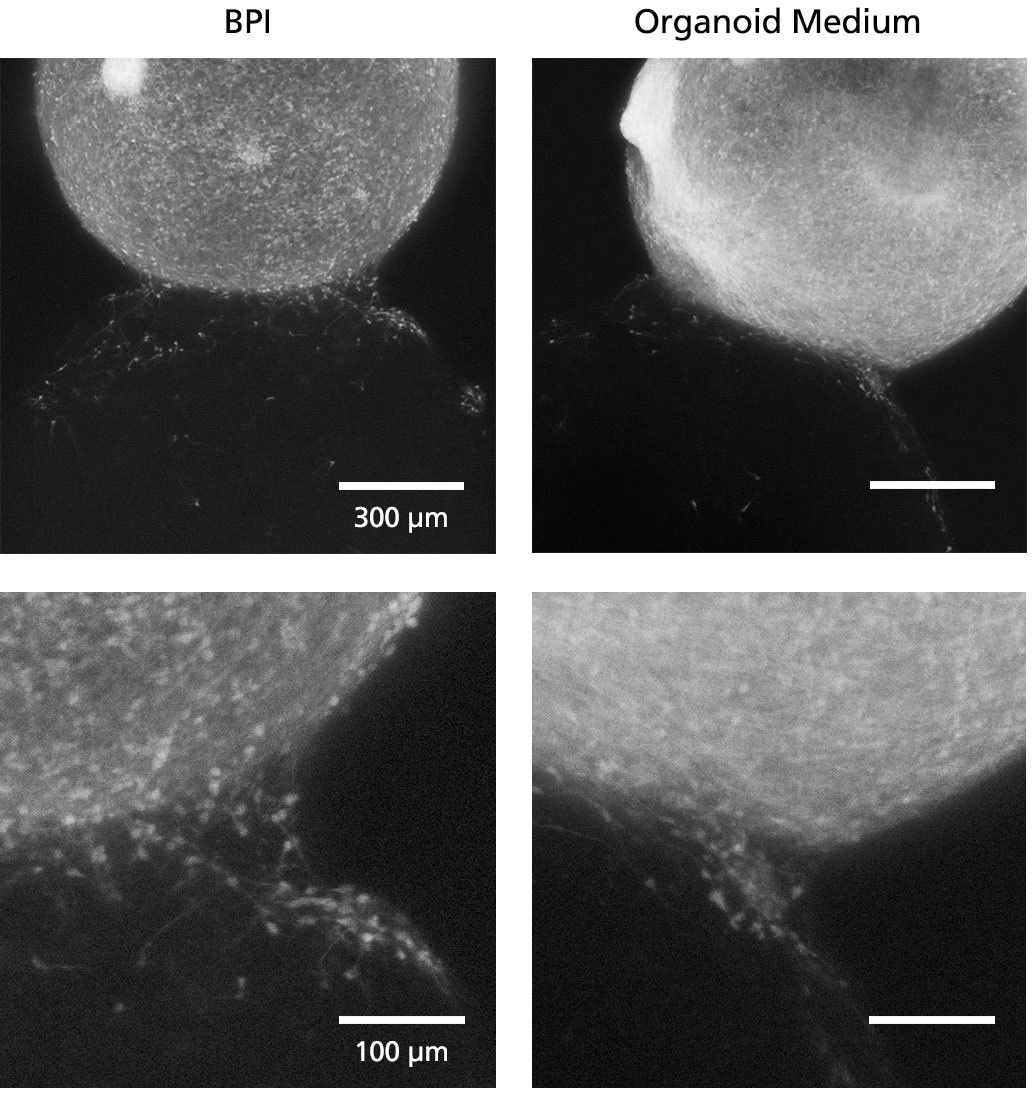
Figure 5. Fluorescent Imaging in BrainPhys™ Imaging Optimized Medium Improves Signal-to-Background Ratios of 3D Neural Cultures
GFP-labeled ventral forebrain organoids were co-cultured and fused with unlabeled dorsal forebrain organoids for one week prior to live imaging in Forebrain Organoid Differentiation Medium from STEMdiff™ Dorsal Forebrain Organoid Differentiation Kit (right) or BrainPhys™ Imaging Optimized Medium (BPI, left). Interneuron migration can be visualized more clearly in BPI. Adapted from Zabolocki et al. 2020, Nature Communications, available under a Creative Commons 4.0 License.
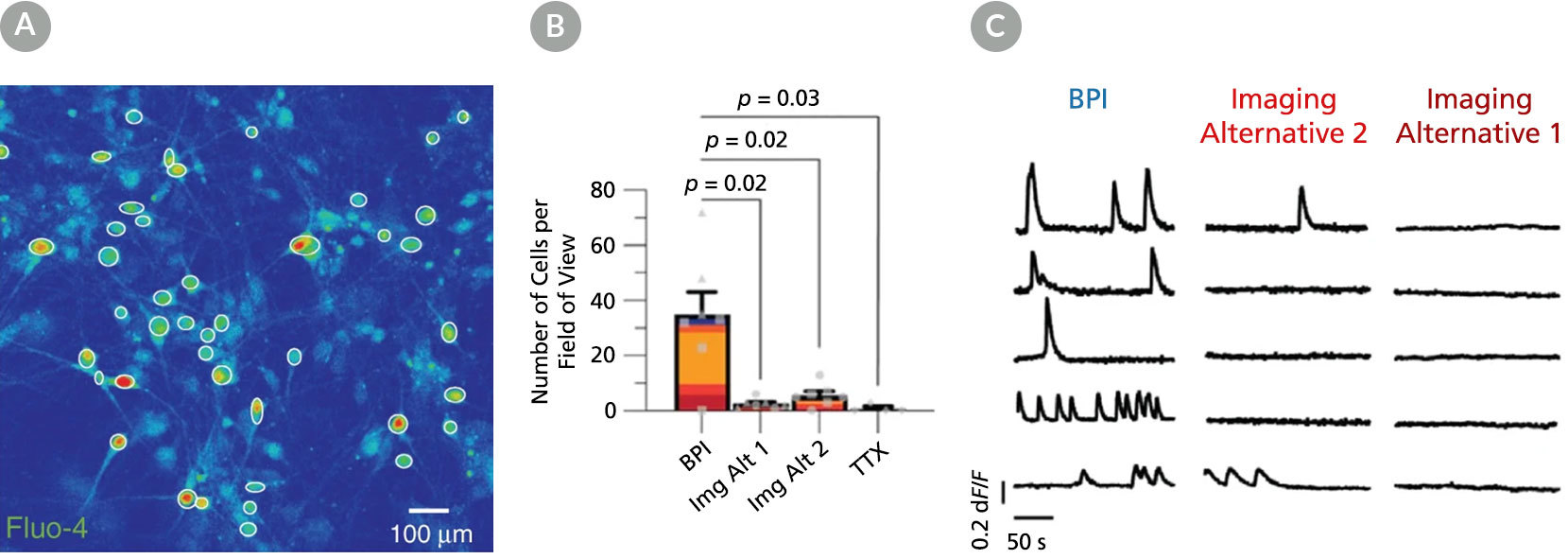
Figure 6. BrainPhys™ Imaging Optimized Medium Supports Live Calcium Imaging of Human Neurons In Vitro
Intracellular Ca2+ changes in human PSC-derived neurons were measured with time-lapse imaging sequences of a Ca2+ sensor. Regions of interest (ROIs) were drawn on the cell soma to determine fluorescence intensity changes (ΔF/F0) over time. The same fields of view (FOVs) were imaged in artificial cerebrospinal fluid (ACSF), BrainPhys™ Imaging Optimized (BPI), Imaging Alternative 2, and Imaging Alternative 1 media. (A) Representative image of a neuronal population in BPI. White circles represent active ROIs, and fluorescent intensity represents intracellular Ca2+ levels. (B) Comparison of Ca2+ signals in BPI, Imaging Alternative 1 (Img Alt 1), and Imaging Alternative 2 (Img Alt 2) conditions show significant differences in the proportions of cells with Ca2+ spikes (two-tailed Mann-Whitney tests, mean ± SEM). Active cells with Ca2+ spikes in BPI (n = 243 cells), Imaging Alternative 2 (n = 39 cells), and Imaging Alternative 1 (n = 18 cells) were compared across seven FOVs from two coverslips. (C) Example traces from the same ROIs in different media. Adapted from Zabolocki et al. 2020, Nature Communications, available under a Creative Commons 4.0 License.
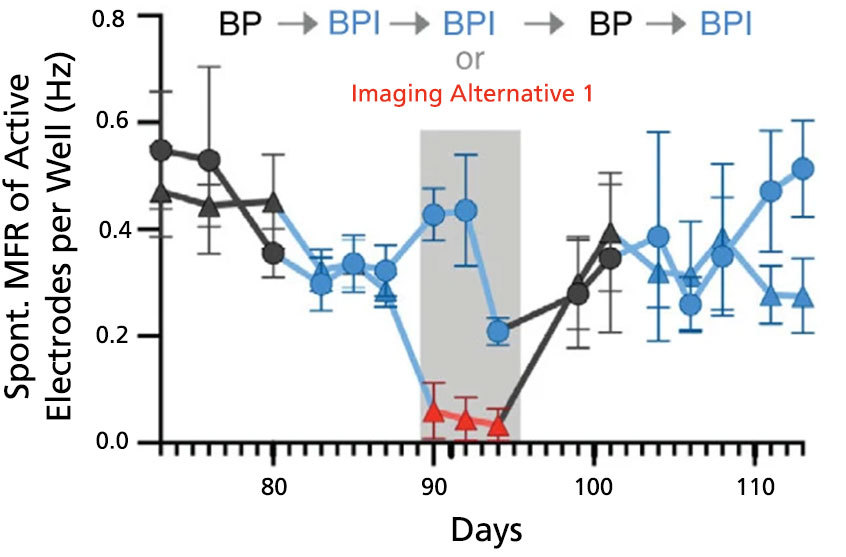
Figure 7. BrainPhys™ Imaging Optimized Medium Supports Long-Term Studies of Neuronal Activity Combined with Imaging-Based Characterization
Spontaneous firing rates of human neurons expressing a synapsin:ChETA-YFP optogene were recorded in BrainPhys™ (BP), BrainPhys™ Imaging Optimized (BPI), or Imaging Alternative 1 media with identical supplements using microelectrode arrays. Recordings were split into two groups (circles, n = 8 wells; or triangles, n = 7 wells). Both groups were switched from BP to BPI after 82 days, and only the triangle group was switched to Imaging Alternative 1 from day 89 - 95. From day 96, both groups were cultured in BP for one week before switching to BPI. Long-term firing is enhanced in BPI relative to Imaging Alternative 1, and mean firing rates (MFR, mean ± SEM) from both groups perform at baseline following the media change at day 96. Adapted from Zabolocki et al. 2020, Nature Communications, available under a Creative Commons 4.0 License.
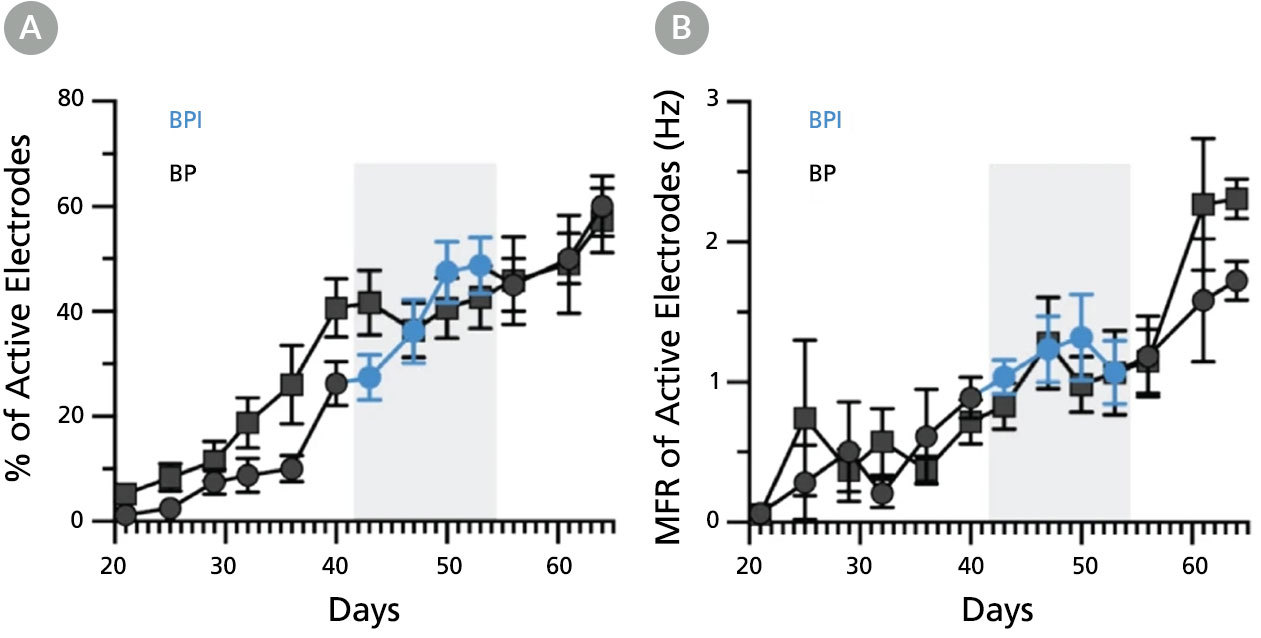
Figure 8. BrainPhys™ Imaging Optimized Medium Supports Long-Term Studies of Human Neuronal Function In Vitro with Equivalent Performance to BrainPhys™ Medium
Multielectrode array (MEA) recordings from human pluripotent stem cell (hPSC)-derived neurons cultured in a 48-well MEA plate in BrainPhys™ (BP) for 9 weeks (n = 6 wells, 96 electrodes) or switched to BrainPhys™ Imaging Optimized (BPI) from BP during weeks 6 - 8 (n = 5 wells, 80 electrodes). (A) Percentage of active electrodes (>0.017Hz) and (B) mean firing rate (MFR) were both maintained during the period in BPI. Values are presented as mean ± SEM. Adapted from Zabolocki et al. 2020, Nature Communications, available under a Creative Commons 4.0 License.