STEMdiff™ Gastric Organoid Differentiation Kit
This kit’s simple, four-stage protocol, based on publications by McCracken et al. (Nature 2014) and Bartfeld et al. (Gastroenterology 2015), enables the generation and differentiation of gastric organoids directly from hPSCs as well as the long-term expansion and cryopreservation of gastric organoids. Organoids cultured with this kit exhibit a cellular composition and organization that recapitulates that of the developing gastric epithelium and associated mesenchyme, providing a physiologically relevant model system for gastric research, as well as drug discovery and compound screening. This kit is optimized for differentiation of cells maintained in mTeSR™1 (Catalog #85850) or mTeSR™ Plus (Catalog #100-0276)
STEMdiff™ Gastric Organoid Differentiation Kit
Culture medium kit for differentiation of human gastric organoids
1 Kit
Catalog # 100-0475
STEMdiff™ Gastric Organoid Expansion Medium
Culture medium kit for differentiation of human gastric organoids
1 Kit
Catalog # 100-0490
⦁ Robust medium supports efficient differentiation of human ES and iPS cells to gastric organoids
⦁ Convenient model system that can be maintained long term through passaging or cryopreserved for experimental flexibility
⦁ Serum-free media system optimized for low experimental variability
- STEMdiff™ Endoderm Basal Medium, 100 mL
- STEMdiff™ Definitive Endoderm Supplement CJ (100X), 1.1 mL
- STEMdiff™ Gastrointestinal Supplement PK, 0.64 mL
- STEMdiff™ Posterior Foregut Supplement, 0.64 mL
- STEMdiff™ Gastric Organoid Medium, 100 mL
- STEMdiff™ Gastric Organoid Expansion Supplement, 1.1 mL
Scientific Resources
Product Documentation
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | All | English |
| Product Information Sheet | STEMdiff™ Gastric Organoid Expansion Medium | 100-0490 | All | English |
| Manual | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | All | English |
| Safety Data Sheet 1 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 2 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 3 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 4 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 5 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 6 | STEMdiff™ Gastric Organoid Differentiation Kit | 100-0475 | 全部 | English |
| Safety Data Sheet 1 | STEMdiff™ Gastric Organoid Expansion Medium | 100-0490 | 全部 | English |
| Safety Data Sheet 2 | STEMdiff™ Gastric Organoid Expansion Medium | 100-0490 | 全部 | English |
Educational Materials (45)
Product Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Data and Publications
数据
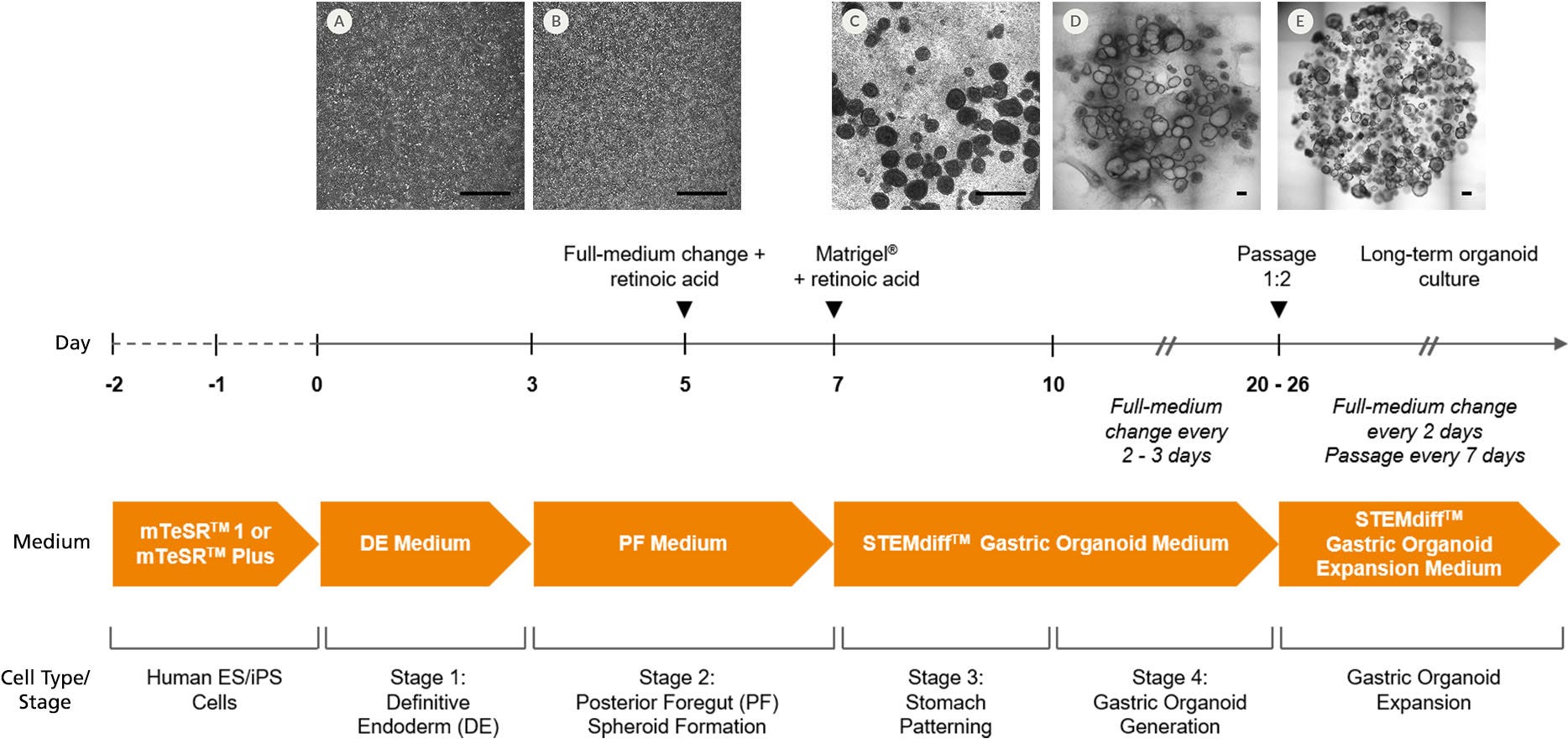
Figure 1. Generation of Human Gastric Organoid Cultures Using STEMdiff™ Gastric Organoid Differentiation Kit
hPSCs were seeded as small aggregates (50 - 200 μm) at low density (4000 aggregates/well) in mTeSR™1 or mTeSR™ Plus on Corning® Matrigel®-coated 24-well plates and allowed to attach overnight. Two-dimensional (2D) monolayer cultures were maintained with daily mTeSR™1 medium changes until a near-confluent monolayer (85 - 90%) was achieved. (A) On day 0, differentiation was initiated by replacing the medium with STEMdiff™ Definitive Endoderm (DE) Medium (Stage 1), then daily medium changes were performed. (B) On day 3, DE Medium was removed and replaced with STEMdiff™ Gastric Posterior Foregut (PF) Medium (Stage 2). On day 5, retinoic acid (RA) was added to PF Medium. (C) On day 7 of differentiation, floating posterior foregut spheroids were harvested from the supernatant and embedded into Corning® Matrigel®. Between days 7 and 10, embedded PF spheroids were cultured in STEMdiff™ Gastric Organoid Medium + RA (Stage 3). Between days 10 and 26, spheroids were matured to gastric organoids surrounded by mesenchyme in STEMdiff™ Gastric Organoid Medium. (D) Between days 20 and 26, gastric organoids were passaged for full differentiation in STEMdiff™ Gastric Organoid Medium until expression of gastric markers was observed (~day 34) and/or (E) expanded in STEMdiff™ Gastric Organoid Expansion Medium to be used for downstream applications or cryopreserved for future experiments. Scale bars = 500 μm.
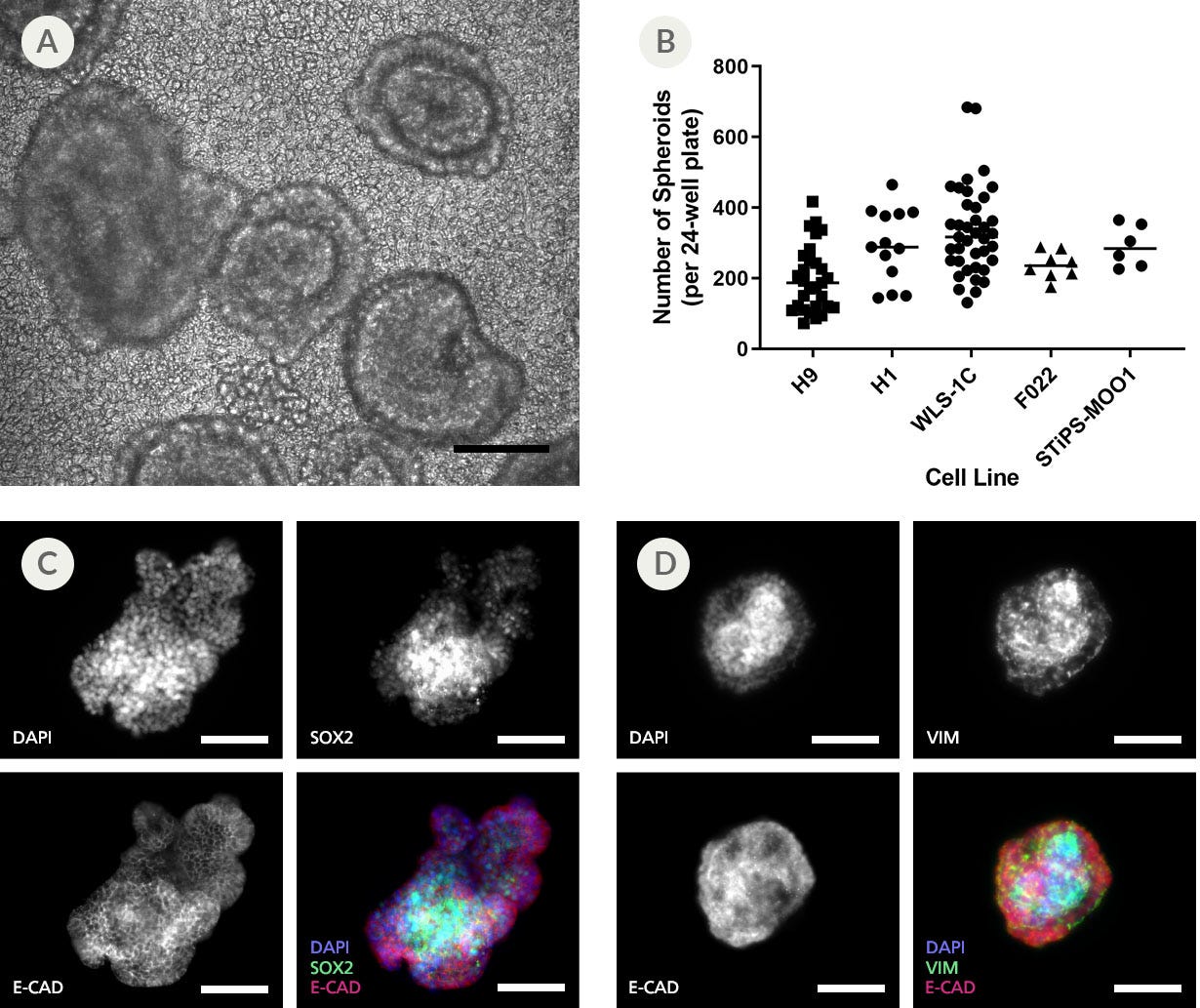
Figure 2. Efficient Formation and Budding of 3D Spheroids From 2D Posterior Foregut Monolayers
The formation and detachment of posterior foregut spheroids from differentiated 2D monolayers was observed between days 5 and 7 of differentiation using STEMdiff™ Gastric Organoid Kit. (A) Representative bright-field microscopy of day 7 spheroid morphologies presenting an outer polarized epithelium surrounding an inner cell mass. (B) Both ESC and iPSC-derived cultures demonstrate efficient spheroid formation upon posterior foregut formation. The total number of spheroids obtained per plate in a given differentiation is shown. (C) Immunofluorescence analysis of released posterior foregut spheroids after 7 days of differentiation. Spheroids express epithelial markers SOX2 and CADHERIN, and (D) a mesenchyme expressing vimentin, but not E-CADHERIN. Scale bars = 100 μm.
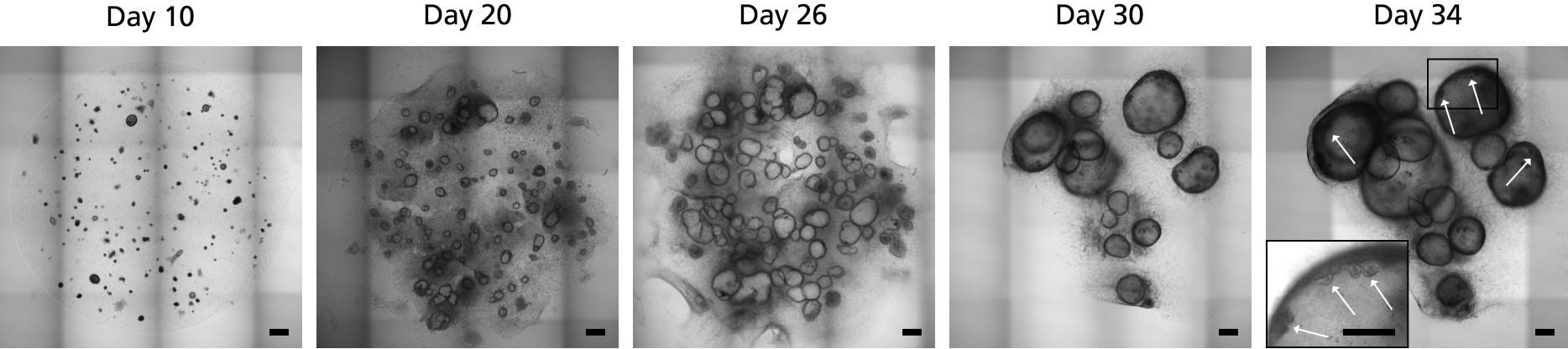
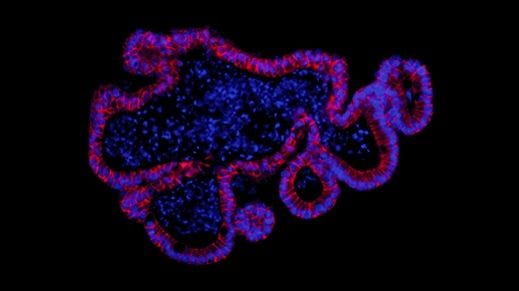
Figure 3. Maturation and Differentiation of Human Gastric Organoids using STEMdiff™ Gastric Organoid Kit
Embedded PF spheroids shown in the image from day 10 were cultured in STEMdiff™ Gastric Organoid Medium and matured into gastric organoids surrounded by mesenchyme (day 20 - 26). Human gastric organoids were fully differentiated in STEMdiff™ Gastric Organoid Medium (day 26 - 34). Differentiated organoids showed a characteristic cystic morphology surrounded by mesenchymal cells. On day 34, formation of buds in the epithelium facing the lumen of the organoid was observed (white arrows). Scale bars = 500 μm.
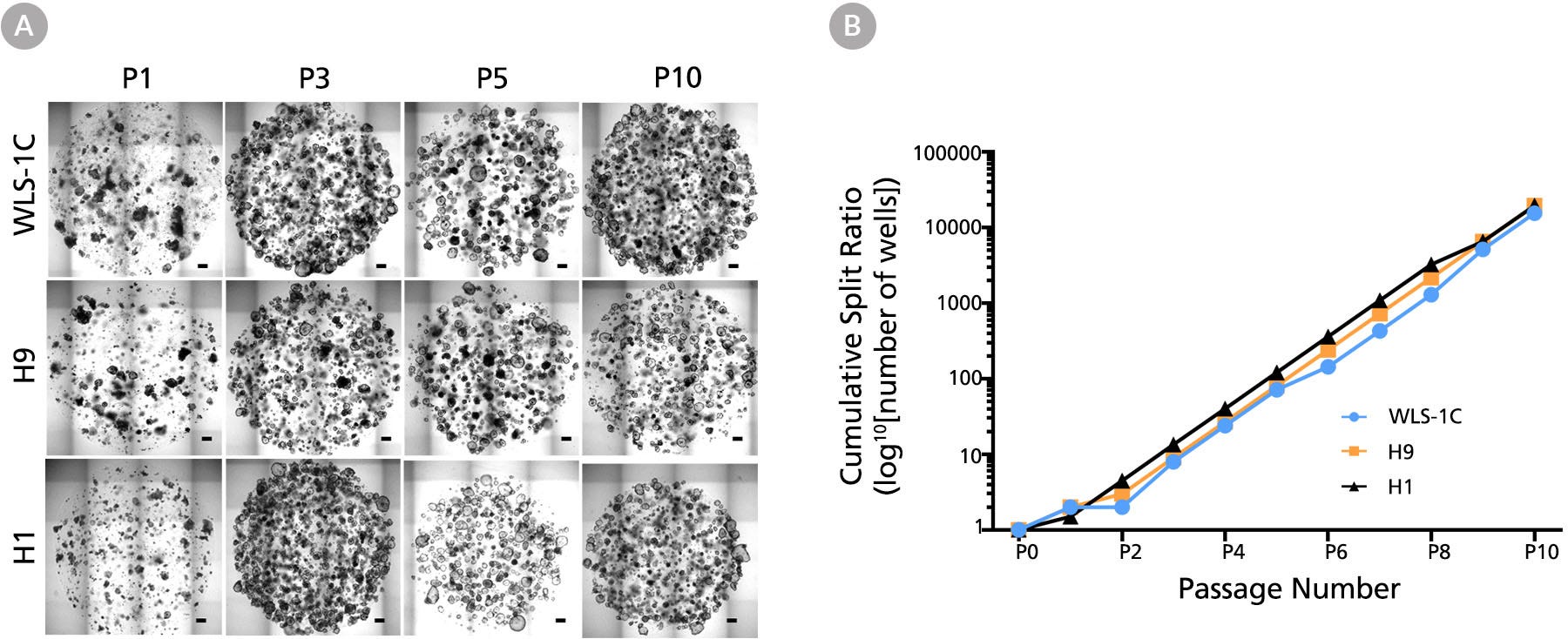
Figure 4. Human Gastric Organoids Expand Rapidly in STEMdiff™ Gastric Expansion Organoid Medium
(A) Human gastric organoids derived from three cell lines were repeatedly passaged every 7 days in STEMdiff™ Gastric Organoid Expansion Medium. Representative images of human gastric organoids derived from WLS-1C iPS, H9 ES, and H1 ES cell lines at the end of passages 1, 3, 5, and 10 are shown. Matrigel® domes contained multiple organoids enriched in epithelial components with passaging progression, while losing the mesenchymal characteristic of early passages. Passage numbers are indicated at the top. Scale bars = 500 μm. (B) Human gastric organoids derived from multiple human ES (H1 & H9) and iPS (WLS-1C) cell lines undergo rapid expansion when maintained in STEMdiff™ Gastric Expansion Medium (n=1).
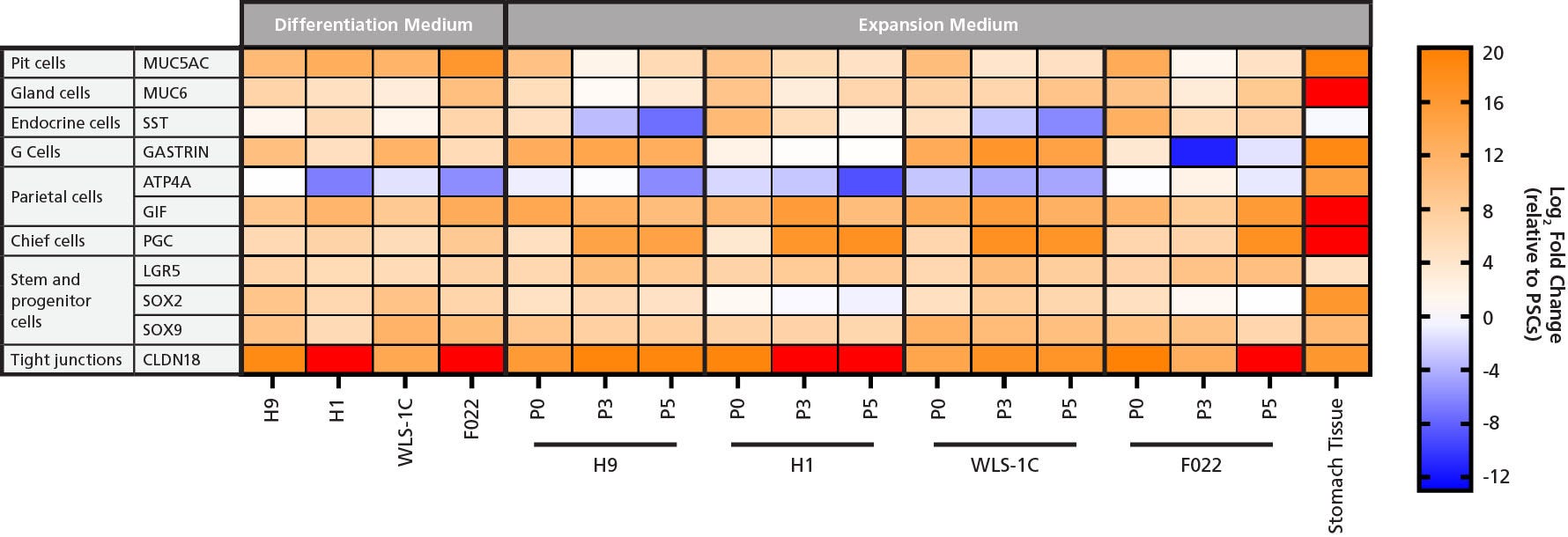
Figure 5. Gene Expression Analysis of Human Gastric Organoids Cultured in Gastric Differentiation and Expansion Medium Shows that Organoids Express Gastric Markers
Gene expression analysis of human gastric organoids derived from four different cell lines (WLS-1C iPS, H9 ES, H1 ES, F022 iPS) cultured in either Differentiation Medium (left) for 34 days or Expansion Medium (right) for 0, 3, or 5 passages demonstrates comparable high expression of tight junction marker CLDN18, stem and progenitor cells markers LGR5, SOX2, and SOX9, and gland cell marker MUC6. High expression levels of MUC5AC and SST in differentiated and P0 organoids indicates the presence of pit and endocrine cells. Downregulation of these two markers was observed with extended culture and expansion to P3 and P5. High expression levels of GIF in both conditions indicates the presence of parietal cells that were not fully matured, as they lacked the expression of ATP4A. The presence of chief cell marker PGC was confirmed in differentiated and P0 organoids, and upregulation in organoids at P3 and P5 suggests enrichment of chief cells in expansion conditions. High expression levels of GASTRIN were observed in differentiated organoids and in human gastric organoids derived from WLS-1C and H9 lines in Expansion Medium; human gastric organoids derived from H1 and F022 show downregulation of GASTRIN in expansion conditions, suggesting a certain degree of inter-cell line variability in differentiating G cells. A commercially available mRNA sample of human adult stomach was used as a positive control. All samples were compared to undifferentiated hPSCs to calculate the relative gene expression (mean ± SD, n=1). hPSC line used and passage number (P) are given below the array.
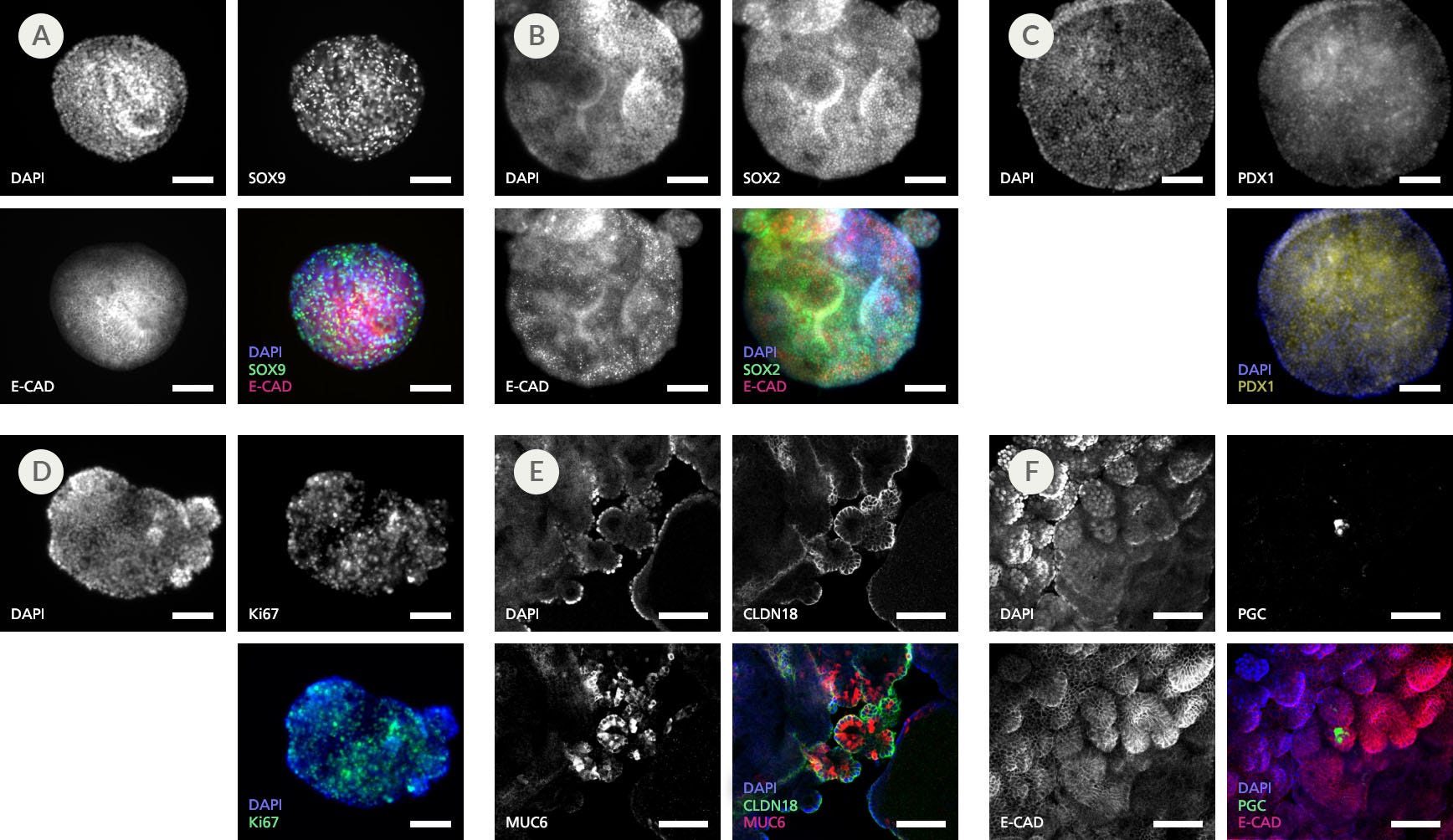
Figure 6. Immunohistochemistry Confirms Expression of Gastric-Specific Markers in Human Gastric Organoids Cultured in Gastric Organoid Expansion Medium
Representative organoids in Expansion Medium at passage 5 expressed progenitor markers (A) SOX9, (B) SOX2, and (C) PDX1; (A,B&F) epithelial marker E-CADHERIN; (D) marker of proliferation Ki67; and (E) gastric tight junction marker CLDN18. (E) Presence of gland cells was detected by expression of MUC6 in the gland regions of the organoids. (F) Detection of scattered expression of PGC indicates differentiation of chief cells (n=2 - 5). Scale bars = 100 μm.
Quality Statement:
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.