mTeSR™ Plus
To enhance cell-quality attributes during restricted feeds, critical medium components have been stabilized, including FGF2, and medium pH is more consistent. As a result, mTeSR™ Plus allows for greater cell expansion rates with daily feeding, while also maintaining cell quality during restricted feeding schedules.
mTeSR™ Plus is compatible with a variety of culture matrices, including Corning® Matrigel® hESC-Qualified Matrix (Corning Catalog #354277) and Vitronectin XF™ (Catalog #07180, a matrix developed and manufactured by Nucleus Biologics).
Each lot of mTeSR™ Plus 5X Supplement is used to prepare complete mTeSR™ Plus medium and then performance-tested in a culture assay using human pluripotent stem cells (hPSCs).
mTeSR™ Plus (Catalog #100-0276) is manufactured under relevant cGMPs, ensuring the highest quality and consistency for reproducible results. For additional quality information, visit www.STEMCELL.com/compliance.
⦁ Supports superior culture morphology and cell growth characteristics
⦁ Enables heightened single-cell survival when used with CloneR™
⦁ Fully compatible with established genome editing and differentiation protocols
- mTeSR™ Plus Kit (Catalog #05825)
- mTeSR™ Plus Basal Medium, 400 mL
- mTeSR™ Plus 5X Supplement, 100 mL
- mTeSR™ Plus Kit, cGMP (Catalog #100-0276)
- mTeSR™ Plus Basal Medium, 400 mL
- mTeSR™ Plus 5X Supplement, 100 mL
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | mTeSR™ Plus | 100-0276 | All | English |
| Manual 1 | mTeSR™ Plus | 05825 | All | English |
| Manual 2 | mTeSR™ Plus | 05825, 100-0276 | All | English |
| Safety Data Sheet 1 | mTeSR™ Plus | 100-0276 | All | English |
| Safety Data Sheet 2 | mTeSR™ Plus | 100-0276 | All | English |
Data
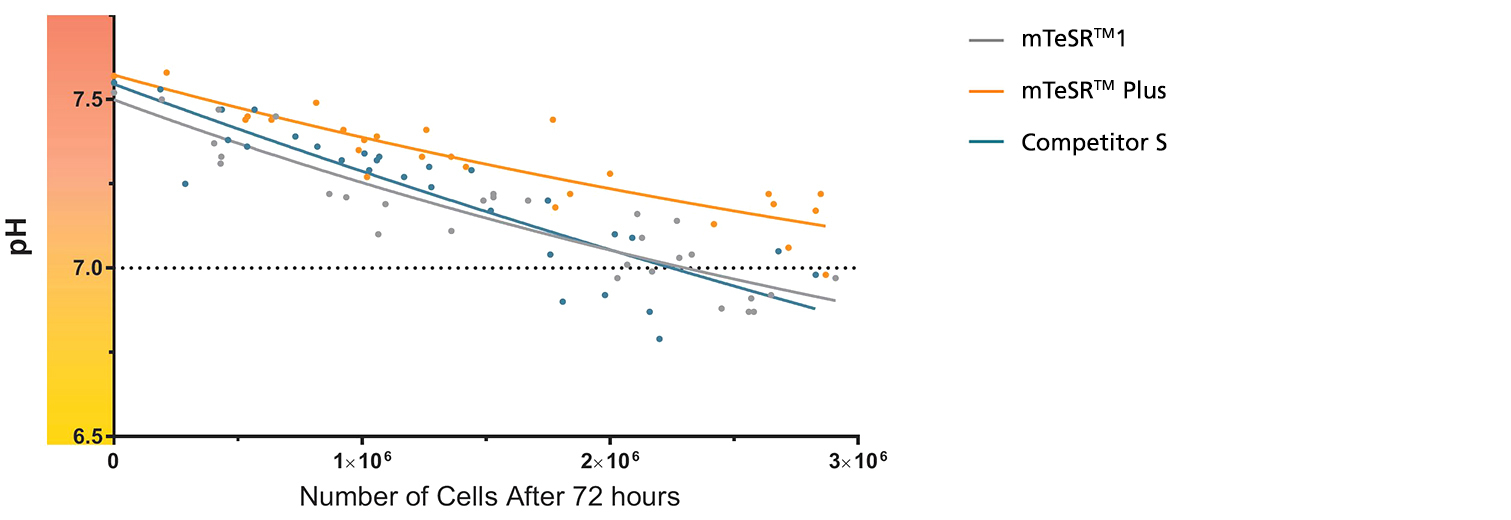
Figure 1. mTeSR™ Plus Maintains Optimal pH Levels Throughout a Weekend-Free Protocol
The pH of spent medium from hPSCs cultured in mTeSR™ Plus is higher than that of hPSCs cultured in mTeSR™1 and other flexible-feeding medium at similar cell densities. pH and cell numbers were measured after a 72-hour period without feeding. Range of cell numbers shown represent different densities that would be observed throughout a typical passage. This demonstrates that feeds can be skipped for two days at any time during routine maintenance using mTeSR™ Plus while maintaining a pH above 7.0. Note: Cultures were fed double the standard medium volume prior to the 72-hour period without feeds in all media and cell numbers are from one well of a 6-well plate.
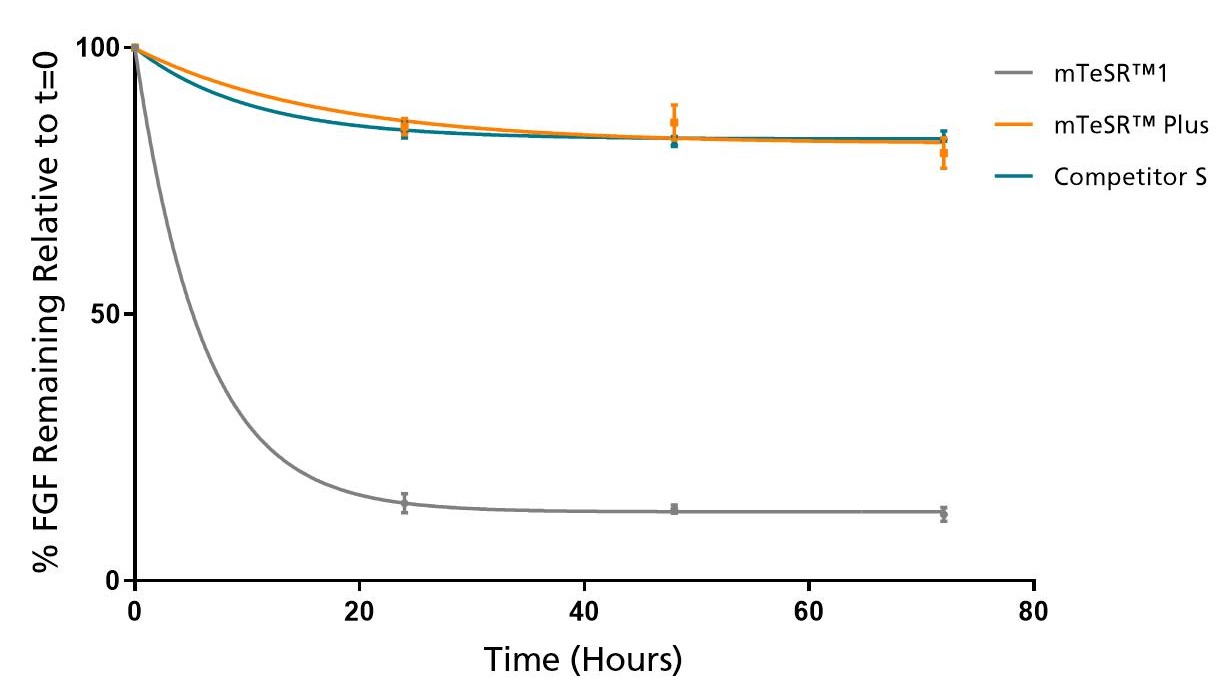
Figure 2. mTeSR™ Plus Maintains Consistent Levels of FGF2 Throughout a Weekend-Free Protocol
FGF2 levels remain high in mTeSR™ Plus when cultured at 37°C over a 72 hour time period. Measured by ELISA.
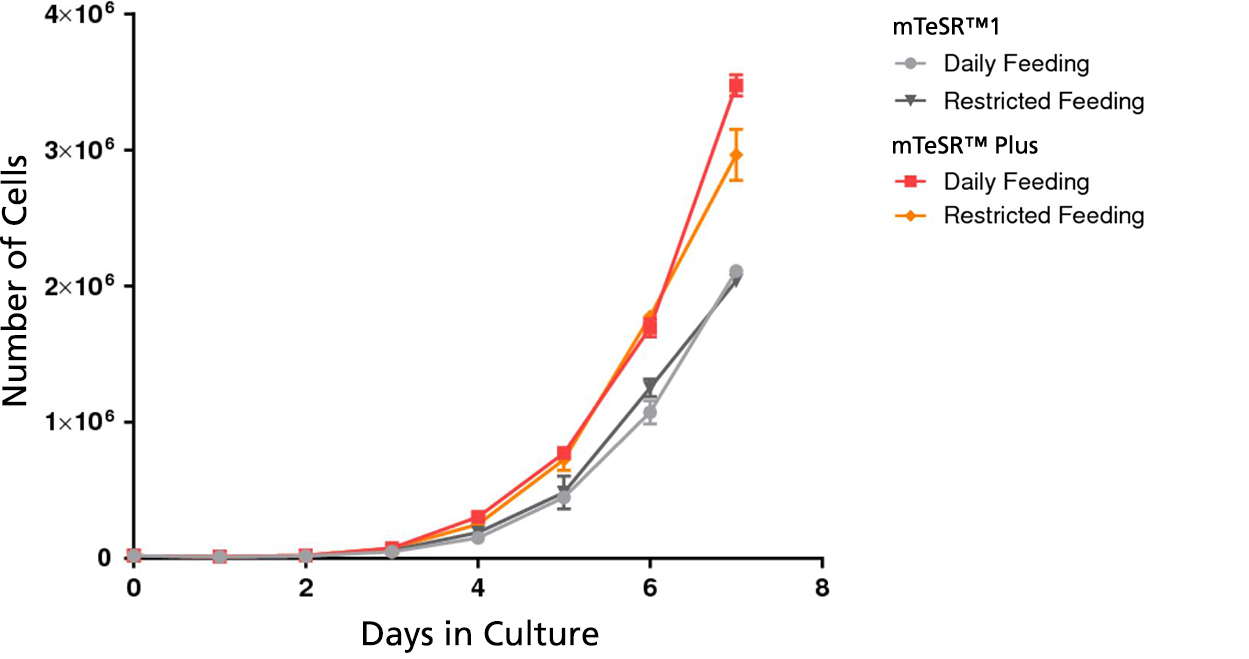
Figure 3. mTeSR™ Plus Supports Higher Cell Numbers
Growth curves were obtained for human ES (H9) cells cultured in mTeSR™1 or mTeSR™ Plus on Corning® Matrigel® matrix over 7 days with either daily feeds or restricted feeds. Growth curves were determined by seeding 20,000 cells per well of a 6-well plate as aggregates and counting the cell numbers each day in duplicate wells.
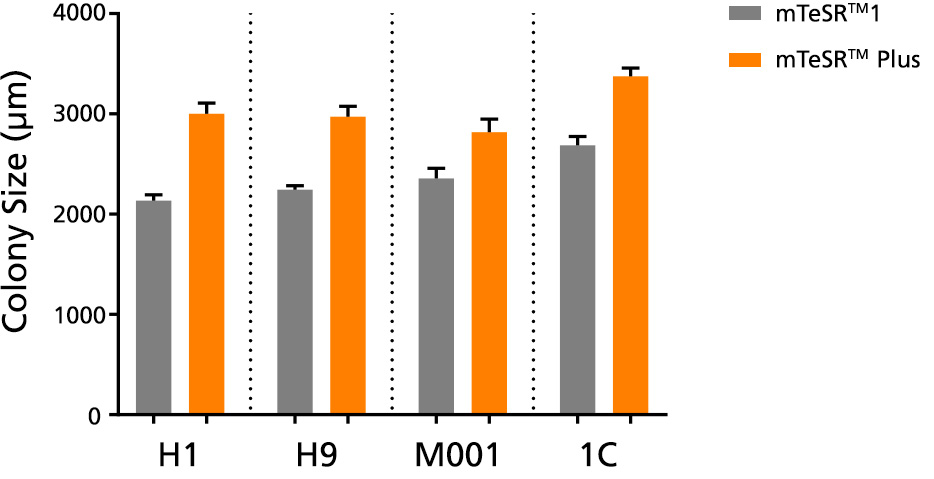
Figure 4. Larger Colonies are Observed in mTeSR™ Plus Cultures
The average colony size per passage (± SEM) was obtained for human ES (H1, H9) and iPS (STiPS-M001, WLS-1C) cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) on Corning® Matrigel® over 10 passages. Size was determined by measuring representative colony diameters at harvest. Note that this data is representative of cultures passaged at a 7-day passaging interval; smaller colony size should be expected if using shorter passaging intervals.
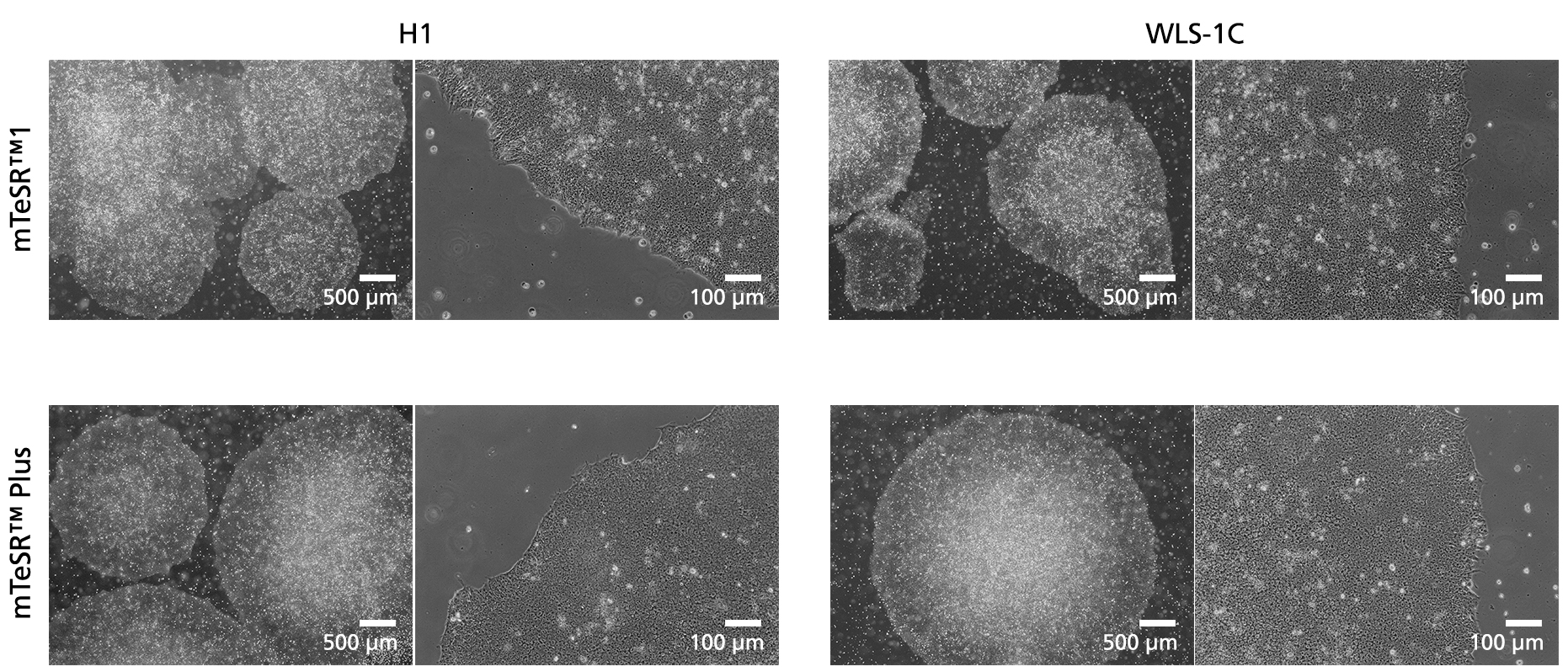
Figure 5. Normal human ES and iPS Cell Morphology is Observed in mTeSR™ Plus Cultures
Images depict undifferentiated human hES (H1) and iPS (WLS-1C) cells cultured on Corning®️ Matrigel®️ matrix in mTeSR™1 with daily feeds or mTeSR™ Plus with restricted feeds. Cells retain the prominent nucleoli and high nuclear-to-cytoplasmic ratio characteristic of this cell type after 10 passages. Densely packed cells and multi-layering are prominent when cells are ready to be passaged.
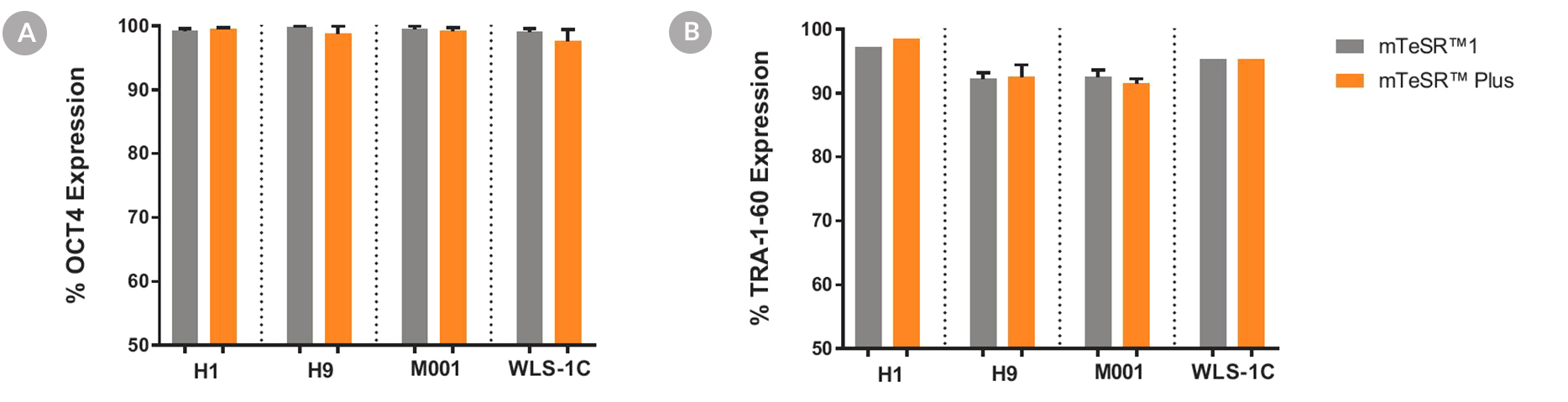
Figure 6. Cells Cultured in mTeSR™ Plus Medium with Restricted Feeding Express Undifferentiated Cell Markers
Human ES (H1, H9) and iPS (WLS-1C, STiPS-M001) cells were characterized using flow cytometry for undifferentiated cell markers, (A) OCT3/4 and (B) TRA-1-60. Graphs show average expression (± SEM) results from analyses of duplicate wells every 5 passages for up to 10-15 passages in mTeSR™1 (daily feeds), or mTeSR™ Plus (restricted feeds).
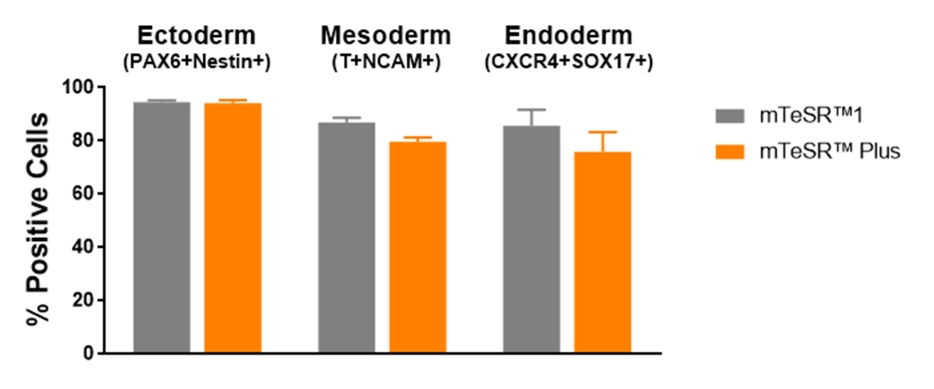
Figure 7. Cells Maintained in mTeSR™ Plus with Restricted Feeding Have Comparable Differentiation Efficiencies to Cells Maintained in mTeSR™1
Human ES (H1, H9) and iPS (WLS-1C, STiPS-M001) cells were maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds). Cells were differentiated using directed differentiation protocols and subjected to flow cytometry analysis. Graphs show average expression (± SEM) results from the 4 cell lines. The markers used for flow cytometry for each germ layer are listed in the bar titles.
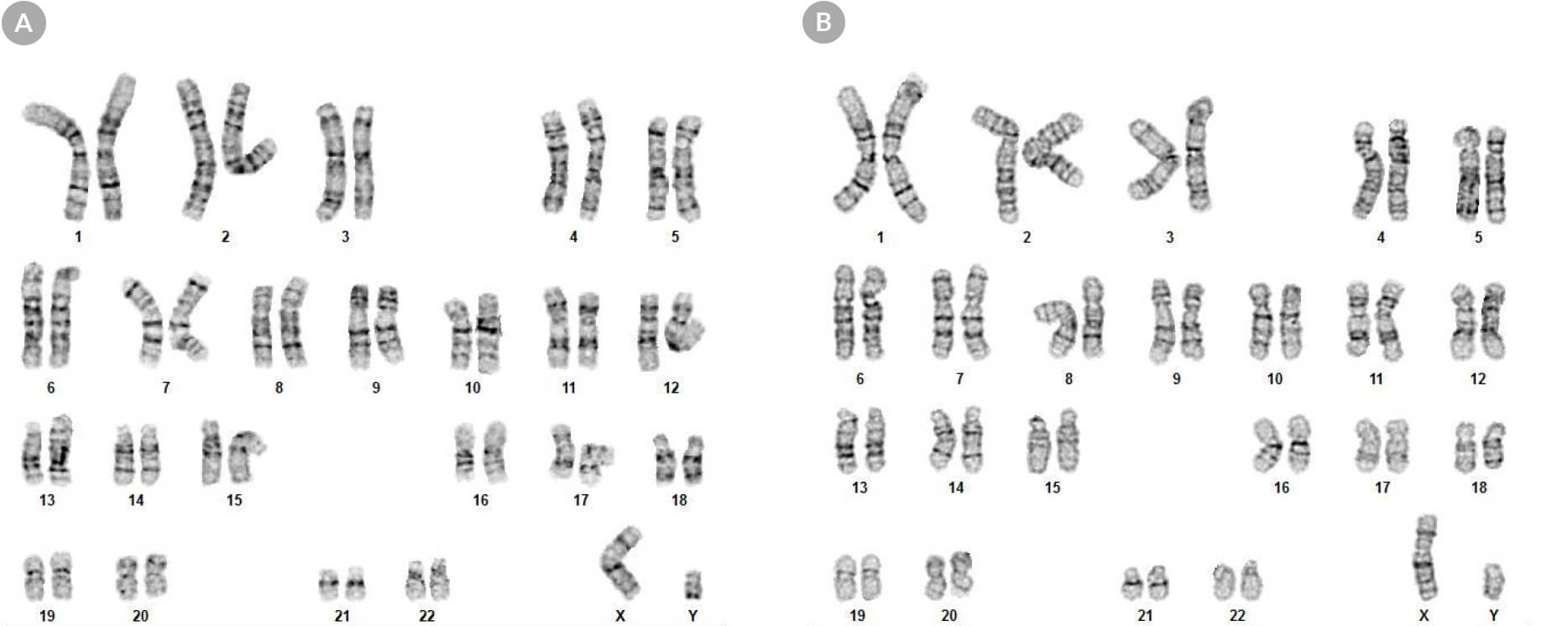
Figure 8. hPSCs Cultured in mTeSR™ Plus with Restricted Feeding Maintain a Normal Karyotype
Karyograms of (A) human ES (H1) and (B) iPS (WLS-1C) cells cultured in mTeSR™ Plus for 30 passages shows a normal karyotype is retained.
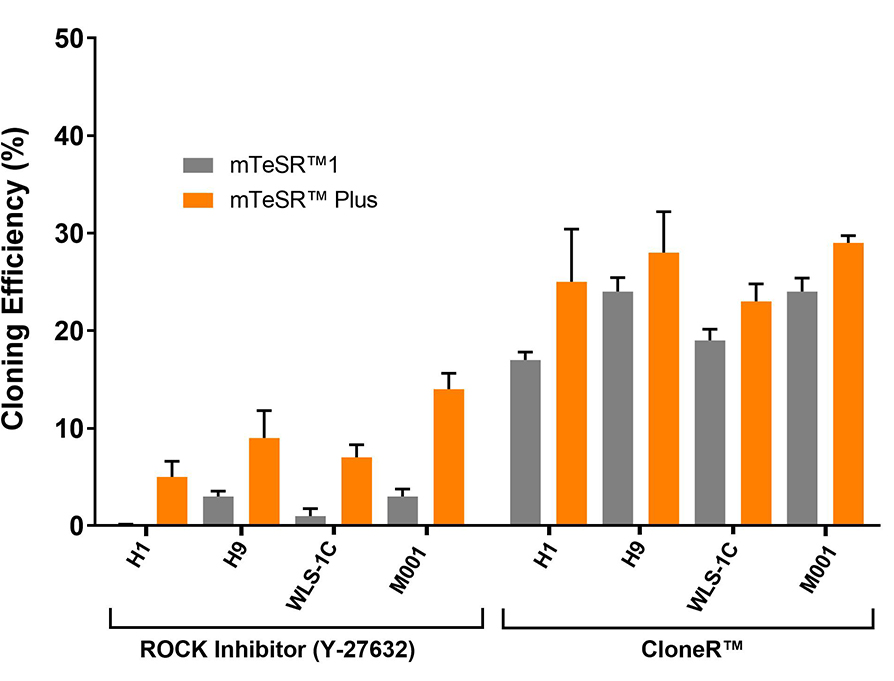
Figure 9. High Cloning Efficiency of hPSCs in mTeSR™ Plus Supplemented with CloneR™
hPSCs (H1, H9, WLS-1C, and STiPS-M001) plated in mTeSR™ Plus with CloneR™ demonstrate cloning efficiencies equal to or greater than hPSCs in mTeSR™1 with CloneR™. Cells were seeded at clonal density (25 cells/cm²) in mTeSR™1 or mTeSR™ Plus on CellAdhere™ Vitronectin™ XF™-coated plates. n ≧ 3 biological replicates.

Figure 10. Representative Cell Morphology 24 Hours After RNP Electroporation in mTeSR™1 and mTeSR™ Plus
H1-eGFP ES cells were plated in (A) mTeSR™1 and (B) mTeSR™ Plus and supplemented with CloneR™ immediately following RNP electroporation. Images were taken 24 hours after electroporation.

Figure 11. Clones Derived in mTeSR™ Plus are Larger and Ready to Be Picked at an Earlier Timepoint
Representative images of human ES (H9) colonies taken 8 days following singlecell plating at clonal density (25 cells/cm²) in either (A) mTeSR™1 or (B) mTeSR™ Plus supplemented with CloneR™ on CellAdhere™ Vitronectin™ XF™-coated plates.
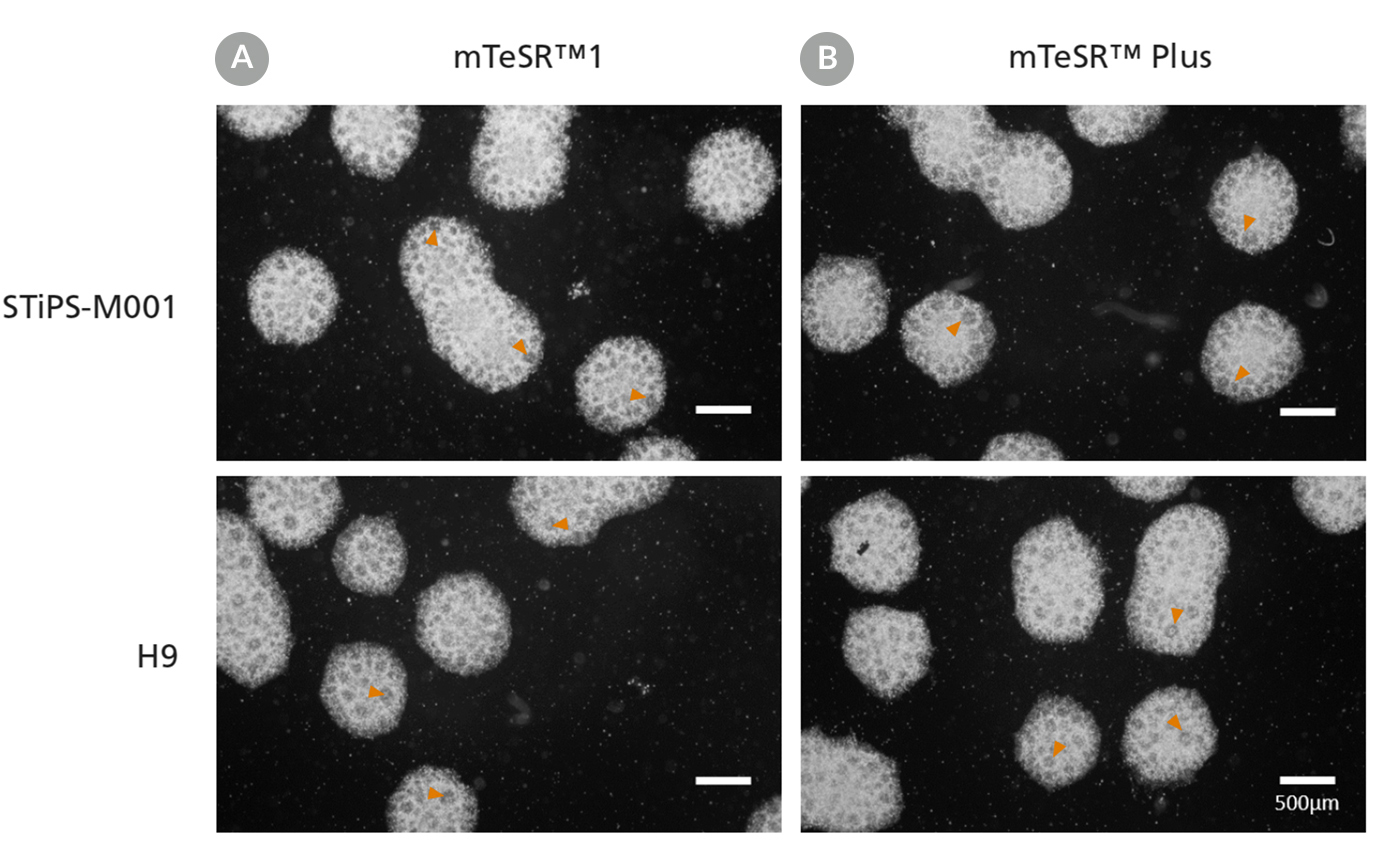
Figure 12. Generation of Neural Progenitor Cells from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) and iPS (STiPS-M001) cells were maintained in (A) mTeSR™1 with daily feeds or (B) mTeSR™ Plus with restricted feeds and differentiated using an embryoid body (EB)-based protocol with STEMdiff™ SMADi Neural Induction Kit. Neural progenitor cells derived from hPSCs maintained in either mTeSR™1 or mTeSR™ Plus clearly display neural rosettes (arrowheads) after replating EBs.

Figure 13. Generation of Cerebral Organoids from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) cells were cultured with mTeSR™ Plus and directed to cerebral organoids using the STEMdiff™ Cerebral Organoid Kit. Image shows apical progenitor marker SOX2 (purple) and neuronal marker TBR1 (green).

Figure 14. Generation of Hematopoietic Progenitor Cells from hPSCs Maintained in mTeSR™ Plus
Human ES (H1, H9) and iPS (STiPS-M001, WLS-1C) cell lines maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) were differentiated to hematopoietic progenitor cells using the STEMdiff™ Hematopoietic Kit. At the end of the differentiation period, cells were harvested from the supernatant and analyzed by flow cytometry for co-expression of CD34+ and CD45+ . (A) Representative density plots showing CD34+ and CD45+ expression, (B) percentage of cells co-expressing CD34+ and CD45+ , and (C) total number of viable cells harvested are shown. Data are expressed as the mean (± SEM); n=4.

Figure 15. Generation of Cardiomyocytes from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) and iPS (WLS-1C) cells were maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) and differentiated to cardiomyocytes using the STEMdiff™ Cardiomyocyte Differentiation Kit. At the end of the differentiation period, cells were harvested and analyzed by microelectrode array (MEA) and flow cytometry. (A) Representative MEA voltage recordings of cardiomyocytes (day 20) demonstrate a characteristic electrical profile and stable beat rate. (B) Percentages of cells expressing cTNT and (C) total number of viable cells harvested are shown. Data are expressed as the mean (± SEM); n=2.

Figure 16. Generation of Intestinal Organoids from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) cells were cultured with mTeSR™ Plus and directed to intestinal organoids using the STEMdiff™ Intestinal Organoid Kit. Image shows markers of the intestinal epithelium EpCAM (green) and CDX2 (red), and intestinal mesenchyme marker vimentin (white). Nuclei are counterstained with DAPI (blue).

Figure 17. Generation of Definitive Endoderm from hPSCs Maintained in mTeSR™ Plus
(A) Representative density plots showing CXCR4 and SOX17 expression in cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds), following 5 days of differentiation using the STEMdiff™ Definitive Endoderm Kit. (B) Quantitative analysis of definitive endoderm formation in multiple hPSC lines (H9, STiPS-M001, WLS-1C) maintained with mTeSR™1 or mTeSR™ Plus as measured by co-expression of CXCR4 and SOX17. Data are expressed as the mean percentage of cells (± SEM) expressing both markers; n=3.

Figure 18. Generation of Pancreatic Progenitors from hPSCs Maintained in mTeSR™ Plus
(A) Representative density plots showing PDX-1 and NKX6.1 expression in cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds), following differentiation using the STEMdiff™ Pancreatic Progenitor Kit. (B) Quantitative analysis of pancreatic progenitor formation in multiple hPS (H9, STiPS-M001, WLS-1C) cell lines maintained with mTeSR™1 or mTeSR™ Plus as measured by co-expression of PDX-1 and NKX6.1. Data are expressed as the mean percentage of cells (± SEM) expressing both markers; n=3.