StemSpan™ SFEM
StemSpan™ SFEM II (Catalog #09605) is an improved version of StemSpan™ SFEM that is further enriched to promote and support higher rates of CD34+ expansion and/or cell differentiation.
• Bovine serum albumin
• Recombinant human insulin
• Human transferrin (iron-saturated)
• 2-Mercaptoethanol
• Supplements
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemSpan™ SFEM | 09600, 09650 | All | English |
| Safety Data Sheet | StemSpan™ SFEM | 09600, 09650 | All | English |
Data

Figure 1. Expansion of CD34 + Human Cord Blood Cells Cultured in StemSpan™ Media Containing CC100 Cytokine Cocktail
Purified CD34 + human cord blood (CB) cells were suspended at a concentration of 10,000 per mL in StemSpan™ SFEM (dark gray bars), SFEM II (gold bars) and ACF (orange bars) media containing CC100 Cytokine Cocktail (Catalog #02690). Cultures were maintained for 7 days, after which the cells were counted and examined for CD34 and CD45 expression by flow cytometry. Shown are the fold expansion of total nucleated cells (TNC) (A) and CD34 + cells (B) per input CD34 + cell, and the percent CD34 + cells (C). Results represent the average results of 32 different CB samples. Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in StemSpan™ SFEM and StemSpan™-ACF (*p<0.001, paired t-test, n=32).

Figure 2. StemSpan™ SFEM II Serum-Free Expansion Medium Containing CC100 Cytokine Cocktail Supports Greater Expansion of Human CD34 + Cells Than Other Media Tested
Expansion of CD34 + cells, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bars) after culturing purified CD34 + CB (A, n=6) or bone marrow (BM) (B, n=3) cells for 7 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, StemPro34 (Life Technologies), X-Vivo-15 and HPGM (both from Lonza), SCGM (Cellgenix), StemLine II (Sigma) and HP01 (Macopharma)). All media were supplemented with StemSpan™ CC100 Cytokine Cocktail (Catalog #02690). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of CB and BM cells produced in StemSpan™ SFEM II were significantly higher than in all other media, except the numbers of CB cells produced in StemSpan™-ACF (*p<0.05, paired t-test).

Figure 3. Expansion of CD34 + Human Cord Blood Cells Cultured in StemSpan™ Media Containing CD34 + Expansion Supplement
Purified CD34 + human cord blood (CB) cells were suspended at a concentration of 10,000 per mL in StemSpan™ SFEM (dark gray bars), SFEM II (gold bars) and ACF (orange bars) media containing CD34 + Expansion Supplement (Catalog #02691). Cultures were maintained for 7 days, after which the cells were counted and examined for CD34 and CD45 expression by flow cytometry. The number of colony-forming units (CFU) in the expanded population was determined by replating cells in MethoCult™ H4435 and counting the number of colonies produced 14 days later. Shown are the fold expansion of total nucleated cells (TNC) (A), CD34 + cells (B) and CFU numbers (C) per input CD34 + cell, and the percent CD34 + cells (D) in these cultures (n=6). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II was significantly higher than in SFEM and ACF (*p<0.001, #p<0.05, paired t-test, n=6).

Figure 4. StemSpan™ SFEM II Serum-Free Expansion Medium Containing CD34 + Expansion Supplement Supports Greater Expansion of Human CD34 + Cells Than Other Media Tested
Expansion of CD34 + cells (A) and CFUs (B), normalized relative to the values obtained in SFEM medium (dark gray bars) after culturing purified CD34 + CB cells for 7 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other suppliers (light gray bars, Competitor 1-6, which included, in random order, X-Vivo-15 (Lonza), HP01 (Macopharma), StemPro34 (Life Technologies), SCGM (Cellgenix), StemLine II (Sigma), and HPGM (Lonza). All media were supplemented with the StemSpan™ CD34 + Expansion Supplement (Catalog #02691). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in all other media (*p<0.01, paired t-test, n=6).
Table 1. Production of Erythroid Cells From CD34 + Human Cord Blood Cells Cultured in StemSpan™ SFEM Serum-Free Expansion Medium Containing Erythroid Expansion Supplement
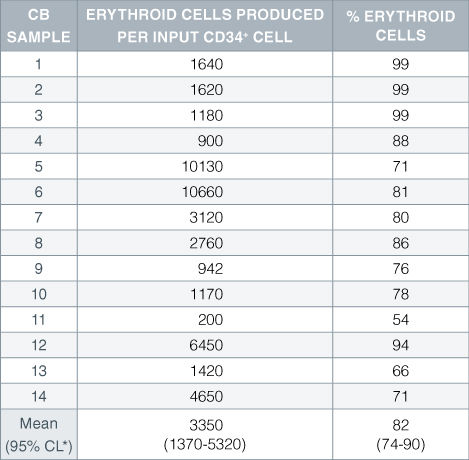
Numbers and percent of erythroid cells produced after 14 days of culture of enriched CD34 + cells from 14 different cord blood (CB) samples. Erythroid cells were characterized by flow cytometry on the basis of transferrin receptor (CD71) and glycophorin A (CD235) expression.*95% confidence limits, the range within which 95% of the results fall.

Figure 5. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Erythroid Expansion Supplement Supports Greater Expansion of Erythroid Cells Than Other Media Tested
The numbers of erythroid cells, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar), obtained after culturing purified CD34 + CB cells for 14 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, X-Vivo-15 and HPGM (both from Lonza), StemLine II (Sigma), HP01 (Macopharma), StemPro34 (Life Technologies) and SCGM (Cellgenix). All media were supplemented with StemSpan™ Erythroid Expansion Supplement (Catalog #02692). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in all other media (*p<0.05, paired t-test, n=6).
Table 2. Production of Megakaryocytes From CD34+ Human Cord Blood Cells Cultured in StemSpan™ SFEM Serum-Free Expansion Medium Containing Megakaryocyte Expansion Supplement
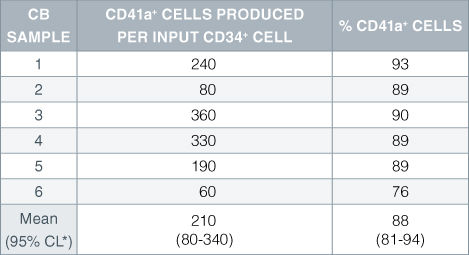
Numbers and percent of cells expressing the megakaryocyte marker CD41a produced after 14 days of culture of enriched CD34 + cells from 6 independent cord blood (CB) samples. *95% confidence limits, the range within which 95% of the results fall.

Figure 6. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Megakaryocyte Expansion Supplement Supports Greater Expansion of Megakaryocytes Than Other Media Tested
The numbers of megakaryocytes, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar), obtained after culturing purified CD34 + CB cells for 14 days in StemSpan™ SFEM, SFEM II (gold bars) and ACF (orange bars), and six media from other commercial suppliers (light gray bars, Competitor 1-6, which included, in random order, StemLine II (Sigma), HPGM (Lonza), HP01 (Macopharma), SCGM (Cellgenix), StemPro34 (Life Technologies) and X-Vivo-15 (Lonza). All media were supplemented with StemSpan™ Megakaryocyte Expansion Supplement (Catalog #02696). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in the StemSpan™ media were significantly higher than in the other media (*p<0.01 paired t-test, n=6).